Abstract
Nitrogen oxides are textbook class of molecular compounds, with extensive industrial applications. Nitrogen and oxygen are also among the most abundant elements in the universe. We explore the N-O system at 0 K and up to 500 GPa though ab initio evolutionary simulations. Results show that two phase transformations of stable molecular NO2 occur at 7 and 64 GPa and followed by decomposition of NO2 at 91 GPa. All of the NO+NO3− structures are found to be metastable at T = 0 K, so experimentally reported ionic NO+NO3− is either metastable or stabilized by temperature. N2O5 becomes stable at 9 GPa and transforms from P-1 to C2/c structure at 51 GPa. NO becomes thermodynamically stable at 198 GPa. This polymeric phase is superconducting (Tc = 2.0 K) and contains a -N-N- backbone.
Similar content being viewed by others
Introduction
Both nitrogen and oxygen have been extensively investigated in experiments and theoretical simulations. Generally, nitrogen is an insulator or a semiconductor. Cubic gauche phase of nitrogen1 is stable in a wide range of pressure2. Other nitrogen structures, such as chain and rings3,4,5, have also been reported. All known phases of oxygen are molecular6,7. Experiments and first-principles calculations for oxygen under high pressure revealed the complex evolution of insulator->semiconductor->metal->semiconductor8. The superconductivity of solid oxygen (Tc = 0.6 K) was observed at pressure above 96 GPa in experiment9. The known nitrogen oxides are semiconducting (for example, the band gap of Im-3 NO2 calculated is approximately 2.8 eV).
At ambient pressure, nitrogen oxides exist as molecular crystals with many applications in chemical industry and important biological roles. The volumetric behavior of nitrous oxide under pressure has been investigated since 196110. The synthesis and phase transformations of N2O have been analyzed in experimental and theoretical studies11,12,13,14,15,16. Different from normal phases containing N2O4 molecules, the ionic NO+NO3− was reported in the range of 1.5 to 3.0 GPa17. The typical N-O stretching frequency of NO+ was characterized at 2234 cm−1, consistent with previous reports17,18. In 2001, Somayazulu et al.19 synthesized the ionic NO+NO3− (nitrosonium nitrate) phase from N2O at above 20 GPa and 1000 K and performed first structural characterization of NO+NO3−. Somayazulu et al.19 proposed an ionic NO+NO3− model based on aragonite with space group of P21cn. Other P21/m20 and Pna2116 models of NO+NO3− were also suggested and the later one is more stable. However, the simulated XRD data of the Pna21 structure is quite different from that in experiments, indicating that other undiscovered stable NO+NO3− structures might exist.
Results and Discussions
We employed the evolutionary algorithm USPEX21,22,23,24 to predict stable N-O compounds and structures under high pressures. Up to 500 GPa, only three stable N-O compounds were found (NO2, N2O5 and NO), as seen in Fig. 1. Most of them retain their molecular structures even under high pressure. Experimentally known “laughing gas” N2O is metastable. The stable phases are discussed as follows.
-
1
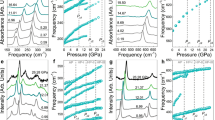
NO2: Besides the known cubic (Im-3) and monoclinic (P21/c) NO2 structures are stable in pressure ranges of 0–7 and 7–64 GPa respectively, another P21/c structure was found to be stable from 64 to 91 GPa (Fig. 2a). Similar to the known phases, this novel NO2 structure also contains N2O4 molecules. Different from known P21/c NO2, the proposed P21/c NO2 is denser and has 8 formula units in the unit cell. NO2 decomposes at 91 GPa.
-
2
N2O5: Molecular N2O5 phases are stable in a wide pressure range (9–446 GPa). At 51 GPa, N2O5 transforms from P-1 (Fig. 2b) to C2/c (Fig. 2c) structure. The N2O5 molecules remain planar. At 446 GPa, N2O5 becomes unstable and decomposes into NO and O. At 0 GPa, the P-1 N2O5 is 0.04 eV/atom more stable than known hexagonal NO2NO3, however, both of them are calculated to be above the convex hull and therefore metastable.
-
3
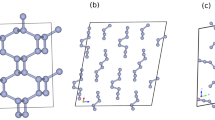
NO: NO is a metastable compound at low pressures. A polymeric NO structure (Fig. 2d, P21/m) becomes stable at 198 GPa. Nitrogen atoms form a strong covalent backbone (N-N = 1.34 Å) in the shape of a zigzag chain. Indeed, that is right between the typical values of single (1.45 Å) and double (1.25 Å) nitrogen-nitrogen bonds. Each nitrogen atom is also bonded to one oxygen atom (N-O bond length is 1.20 Å). A similar backbone has also been reported for the N-H system25. Distance between neighboring quasi-one-dimensional structures is 1.86 Å. Phonon dispersion curves of this remarkable polymeric phase were calculated (shown in Fig. S6). No imaginary frequencies were observed, implying its dynamical stability.
While most of the stable N-O phases are semiconducting, polymeric NO is metallic. The band structure of NO is shown in Fig. 3. Using the Allen-Dynes modified McMillan equation26,27 with value of the Coulomb pseudopotential μ* = 0.13, polymeric NO is superconducting with Tc = 2.0 K at 200 GPa, which is close to that of oxygen8,9.
As mentioned above, ionic NO+NO3- has been observed in several high-pressure experiments19,20,28. However, no stable NO+NO3− structure was found in our variable-composition searches. To find the lowest-enthalpy ionic NO+NO3− structure, we performed (NO)n(NO3)n (n = 6 or 8) calculations at 0–50 GPa, assembling structures from ready-made NO and NO3 units in variable proportion. A novel metastable monoclinic NO+NO3− (P21, Fig. 4) was found to be more stable than orthorhombic phase16 and monoclinic P21/m NO+NO3− 20 at pressures above 1.7 GPa. The main difference between novel P21 and P21/m NO+NO3− models20 is the orientation of the NO+ molecules. Importantly, at all pressures structures made of N2O4 molecules are more stable than ionic NO+NO3− structures (Fig. 4)
In experiments, the typical Raman frequencies of NO+NO3− are 2234 cm−1 for the N-O stretch in NO+, together with 1345, 1056 and 721 cm−1 for anti-symmetric stretch, symmetric stretch and in-plane deformation for NO3− respectively17,19,28,29,30. The Raman frequencies and intensities of NO+NO3− and NO2 structures were calculated at 20 GPa. Here, Raman frequencies of NO+ and NO3− were used for comparison. As shown in Fig. 5, the computed Raman spectra of P21/c NO2 and Pna21 NO+NO3− are significantly different from experimental ones.17. The typical Raman frequencies of N-O stretch are 2071 cm−1 of P21 and 2151 cm−1 of P21/m structures. Both of them basically match the experimental data19, but that of P21 NO+NO3− obtains better match in terms of relative intensity. Similar comparison for Raman and XRD data could also be observed under other pressures20,31.
In summary, stable NO2, N2O5 and NO phases were found in N-O system up to 500 GPa. The P21/c NO2 becomes stable at 64 GPa and decomposes at 91 GPa. N2O5 with P-1 becomes stable at 9 GPa, transforms to C2/c at 51 GPa and decomposes at 446 GPa. The only metallic structure (P21/m NO) has -N-N- zigzag backbone and possesses superconductivity with Tc = 2.0 K. Our results show that ionic NO+NO3− is metastable and we identify a novel P21 structure that matches experimental data better and has lower enthalpy than previously proposed structures.
Methods
An evolutionary algorithm, as implemented in the USPEX code21,22,23,24, were utilized to search for the stable compounds and structures. This method has already been successfully applied to study numerous systems, including nitrogen and oxygen under pressure5,7. Structure relaxations were done using density functional theory (DFT)32,33 within the generalized gradient approximation (GGA)34 using the all-electron projector augmented wave (PAW)35,36 method as implemented in the VASP code37. The plane-wave kinetic energy cutoff was set to 600 eV and Brillouin zone was sampled at a resolution of 2π×0.06 Å−1. At first, variable-composition were carried out at 0, 10, 20, 30, 50, 100, 150, 200, 250, 300, 350, 400, 450 and 500 GPa. Stability of compounds was judged using the convex hull construction: those compounds which are on the convex hull (i.e. which are more favorable than any isochemical mixture of other phases) are thermodynamically stable at given conditions. The PHONOPY code38 was employed to calculate phonon dispersions for all promising structures and all the discussed structures were found to be dynamically stable. All Raman frequencies and intensities were calculated according to the method of Porezag and Pederson39. The electron–phonon coupling calculations in Quantum Espresso40 with 180 Ry plane-wave cutoff energy were used to calculate the critical temperature of superconductivity (Tc).
Additional Information
How to cite this article: Li, D. et al. Nitrogen oxides under pressure: stability, ionization, polymerization and superconductivity. Sci. Rep. 5, 16311; doi: 10.1038/srep16311 (2015).
References
Mailhiot, C., Yang, L. H. & Mcmahan, A. K. Polymeric Nitrogen. Physical Review B 46, 14419–14435 (1992).
Dong, H., Oganov, A. R., Zhu, Q. & Qian, G. R. The phase diagram and hardness of carbon nitrides. Sci Rep 5, 9870 (2015).
Mattson, W. D., Sanchez-Portal, D., Chiesa, S. & Martin, R. M. Prediction of new phases of nitrogen at high pressure from first-principles simulations. Physical Review Letters 93, 125501 (2004).
Yao, Y., John, S. T. & Tanaka, K. Metastable high-pressure single-bonded phases of nitrogen predicted via genetic algorithm. Physical Review B 77, 052103 (2008).
Ma, Y., Oganov, A. R., Li, Z., Xie, Y. & Kotakoski, J. Novel high pressure structures of polymeric nitrogen. Physical Review Letters 102, 065501 (2009).
Akahama, Y., Kawamura, H., Häusermann, D., Hanfland, M. & Shimomura, O. New High-Pressure Structural Transition of Oxygen at 96 GPa Associated with Metallization in a Molecular Solid. Physical Review Letters 74, 4690–4693 (1995).
Ma, Y., Oganov, A. R. & Glass, C. W. Structure of the metallic ζ-phase of oxygen and isosymmetric nature of the ε-ζ phase transition: Ab initio simulations. Physical Review B 76, 064101 (2007).
Sun, J., Martinez-Canales, M., Klug, D. D., Pickard, C. J. & Needs, R. J. Persistence and eventual demise of oxygen molecules at terapascal pressures. Physical review letters 108, 045503 (2012).
Shimizu, K., Suhara, K., Ikumo, M., Eremets, M. & Amaya, K. Superconductivity in oxygen. Nature 393, 767–769 (1998).
Couch, E. J., Kobe, Kenneth. A. Volumetric Behavior of Nitrous Oxide. Pressure-Volume Isotherms at High Pressures. J Chem Eng Data 6, 229–233 (1961).
Agnew, S. F., Swanson, B., Jones, L. & Mills, R. Disproportionation of nitric oxide at high pressure. The Journal of Physical Chemistry 89, 1678–1682 (1985).
Olijnyk, H., Daufer, H., Rubly, M., Jodl, H. J. & Hochheimer, H. D. Effect of Pressure and Temperature on the Raman-Spectra of Solid N2O. Journal of Chemical Physics 93, 45–54 (1990).
Mills, R. L., Olinger, B., Cromer, D. T. & LeSar, R. Crystal structures of N2O to 12 GPa by x-ray diffraction. The Journal of Chemical Physics 95, 5392 (1991).
Yoo, C. S. et al. Disproportionation and other transformations of N2O at high pressures and temperatures to lower energy, denser phases. J Phys Chem B 107, 5922–5925 (2003).
Iota, V., Park, J. H. & Yoo, C. S. Phase diagram of nitrous oxide: Analogy with carbon dioxide. Physical Review B 69 (2004) 10.1103/Physrevb.69.064106.
Xiao, H., An, Q., Goddard, W. A., 3rd, Liu, W. G. & Zybin, S. V. Formation of the -N(NO)N(NO)- polymer at high pressure and stabilization at ambient conditions. Proc Natl Acad Sci USA 110, 5321–5325 (2013).
Agnew, S. F., Swanson, S. I., Jones, L. H., Mills, R. L. & Schlferl, D. Chemistry of N204 at High Pressure: Observation of a Reversible Transformation between Molecular and Ionic Crystalline Forms. Journal of physical and chemical 87, 5065–5068 (1983).
Goulden, J. D. S. & Millen, D. J. Vibrational Spectra of Ionic Forms of Oxides and Oxy-Acids of Nitrogen.6. Raman-Spectral Evidence of the Ionisation of Dinitrogen Tetroxide in Nitric Acid - the Nitrosonium Ion, NO+ and the Nitrosonium - Nitrogen Dioxide Ion, N2O3 +. J Chem Soc 2620–2627 (1950), 10.1039/Jr9500002620
Somayazulu, M. et al. Novel broken symmetry phase from N2O at high pressures and high temperatures. Physical Review Letters 87, 135504 (2001).
Meng, Y. et al. Hard x-ray radiation induced dissociation of N2 and O2 molecules and the formation of ionic nitrogen oxide phases under pressure. Physical Review B 74 (2006), 10.1103/PhysRevB.74.214107
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. Journal of Chemical Physics 124, 244704 (2006).
Oganov, A. R., Ma, Y., Lyakhov, A. O., Valle, M. & Gatti, C. Evolutionary crystal structure prediction as a method for the discovery of minerals and materials. Reviews in Mineralogy and Geochemistry 71, 271–298 (2010).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How Evolutionary Crystal Structure Prediction Works · and Why. Accounts of chemical research 44, 227–237 (2011).
Zhu, Q. & Oganov, A. R., Glass, C. W. & Stokes, H. T. Constrained evolutionary algorithm for structure prediction of molecular crystals: methodology and applications. Acta Crystallographica Section B: Structural Science 68, 215–226 (2012).
Goncharov, A. F. et al. Backbone NxH compounds at high pressures. The Journal of Chemical Physics 142, 214308 (2015).
McMillan, W. L. Transition Temperature of Strong-Coupled Superconductors. Physical Review 167, 331–344 (1968).
Allen, P. B. & Dynes, R. C. Transition temperature of strong-coupled superconductors reanalyzed. Physical Review B 12, 905–922 (1975).
Song, Y. et al. High-pressure stability, transformations and vibrational dynamics of nitrosonium nitrate from synchrotron infrared and Raman spectroscopy. Journal of Chemical Physics 119, 2232–2240 (2003).
Song, Y., Hemley, R. J., Mao, H. K. A., Liu, Z. X. & Herschbach, D. R. New phases of N2O4 at high pressures and high temperatures. Chemical Physics Letters 382, 686–692 (2003).
Song, Y., Somayazulu, M., Mao, H. K., Hemley, R. J. & Herschbach, D. R. High-pressure structure and equation of state study of nitrosonium nitrate from synchrotron x-ray diffraction. Journal of Chemical Physics 118, 8350–8356 (2003).
Sihachakr, D. & Loubeyre, P. High-pressure transformation of N2/O2 mixtures into ionic compounds. Physical Review B 74 (2006).10.1103/Physrevb.74.064113
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Physical review 136, B864 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Physical Review 140, A1133 (1965).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Physical review letters 77, 3865 (1996).
Blöchl, P. E. Projector augmented-wave method. Physical Review B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B 59, 1758 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B 54, 11169 (1996).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Physical Review B 78, 134106 (2008).
Porezag, D. & Pederson, M. R. Infrared intensities and Raman-scattering activities within density-functional theory. Physical Review B 54, 7830–7836 (1996).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. Journal of Physics: Condensed Matter 21, 395502 (2009).
Acknowledgements
The authors appreciate financial supports from Natural science foundation of China (No. 51502098) and Fujian Province (No. 2013J05075) and Fujian Education Department (No. JA13016). The authors would like to thank Prof. Jincao Dai and Qianku Hu for their helpful suggestions.
Author information
Authors and Affiliations
Contributions
D.X.L., X.D., G.R.Q., Q.Z., H.F.D. and X.F.Z. performed calculations and analyzed the data. D.X.L., A.R.O. and Q.Z. wrote the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, D., Oganov, A., Dong, X. et al. Nitrogen oxides under pressure: stability, ionization, polymerization and superconductivity. Sci Rep 5, 16311 (2015). https://doi.org/10.1038/srep16311
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16311
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.