Abstract
Evidence between age at menarche and endometrial cancer risk have been controversial. Therefore, we conducted a meta-analysis of prospective studies to analyze the aforementioned association. Relevant studies were identified by searching PubMed and EMBASE databases until the end of June 2015. A random-effects model was used to estimate summary relative risks (RRs) and 95% confidence intervals (CIs) for associations between menarcheal age and endometrial cancer risk. Our meta-analysis included eight prospective studies involving 4553 subjects with endometrial cancer. The summarized RRs of endometrial cancer for menarcheal age were 0.68 (95%CI = 0.58–0.81, I2 = 41.9%, P = 0.099, n = 8) when comparing women with oldest category of menarcheal age with women with youngest category of menarcheal age. Notably, there was an 4% reduction in risk for per 2 years delay in menarcheal age (summarized RR = 0.96; 95%CI = 0.94–0.98, I2 = 45.7%, P = 0.101, n = 6). Additionally, significant inverse associations were consistent within all stratified analyses. There was no evidence of publication bias or significant heterogeneity between subgroups detected by meta-regression analyses. Our findings support the hypothesis that late menarcheal age is inversely associated with endometrial cancer risk. Further larger prospective or pooled studies are warranted to fully adjust for potential confounders and distinguish whether the associations differ by histological subtypes of endometrial cancer.
Similar content being viewed by others
Introduction
Endometrial cancer is the second most common gynecologic malignancy worldwide, with approximately 280,000 new cases in 20121. Recently, the development of endometrial cancer may be partly attributed to both endogenous and exogenous of estrogen and progesterone2. For example, estrogens are the primary stimulants of endometrial proliferation3, which is a prerequisite for carcinogenesis4. Apparently, unchecked proliferation can lead to malignant transformation5, which follows that estrogens are a cause in the development of at least some endometrial cancer. In contrast, the surge of luteal progesterone secretion which begins just prior to ovulation each month in premenopausal women may play a role in arrest endometrial proliferation and promoting secretory differentiation of the endometrium, which can mitigate against the occurrence of endometrial cancer2,6,7. In 1986, Pettersson et al.8 first suggested that menstruation span was an important risk factor for endometrial cancer. On the basis of this finding, they addressed that the number of ovulatory cycles might be important in the etiology of this disease9. Therefore, reproductive characteristics, such as age at menarche, age at menopause and nulliparity, that are closely associated with endogenous hormone changes may affect the risk of endometrial cancer2.
Menarche was considered to be the milestone of ovulation initiation as well as the initiation of hormone changes in the childhood and adolescent period10. Our previous study found a statistically significant inverse association between later menarcheal age and ovarian cancer risk11. Furthermore, some studies suggested that increasing rate of overweight/obesity in childhood and adolescent period was inverse associated with the menarcheal age12,13,14. In addition, early menarcheal age increased the risk of all-cause or cardiovascular disease mortality in several studies during the past decade15,16,17,18. Although the increased risk of endometrial cancer associated with early menarcheal age has been attributed to a longer lifetime exposure to endogenous estrogen and progesterone deficiency associated with anovulatory cycles19,20,21, the evidence from observational studies are conflicting, which might be partly attributed to different study designs and limited sample sizes of individual studies. Notably, minority of these studies provided the detail information of the risk estimation of per year advance/delay in menarcheal age by dose-response analysis. Therefore, we carried out this meta-analysis of all prospective studies published up to May 2014 to systematically and quantitatively evaluate this issue.
Results
Literature search
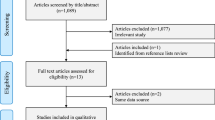
We identified 4607 potentially relevant articles from our search of the MEDLINE (PubMed) and EMBASE databases. Of these, 4593 articles were excluded after the first screening based on the abstract or title, leaving 14 articles for full-text review. On this review, two articles22,23 were excluded because of duplicate reports from the same study population and four articles24,25,26,27 were excluded because they did not report sufficient data of risk estimates. Overall, 8 prospective studies were included in this study (Fig. 1).
Study characteristics
Table 1 summarizes the characteristics of the 8 included studies. These included articles which represent 4,553 cases and 949,945 non-cases, were published between 1988 and 2013 and consist of 7 cohort studies9,21,28,29,30,31,32 and one nested case-control study33. Of the 8 prospective studies, five were conducted in the United States28,29,30,31,33, one each in China9, Norway32 and the European Prospective Investigation into Cancer and Nutrition (EPIC)21. Sample sizes of these included studies ranged from 15,52833 to 301,60121 and the number of endometrial cases varied from 3933 to 145028. The median duration of follow-up was 11 years.
Late versus early age at menarche
The summary RR of endometrial cancer for the oldest versus the youngest categories of menarcheal age was 0.68 (95% CI: 0.58–0.81) with moderate heterogeneity (I2 = 41.9%, P = 0.099) (Table 2, Fig. 2). There was no indication of publication bias with Egger’s test (P-bias = 0.189) and no asymmetry in the funnel plots when inspected visually.
Forest plot (random effects model) of menarcheal age and endometrial cancer risk in prospective studies.
Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk estimate with its 95% CI. CI: confidence interval; RR: relative risk.
Dose-response analysis
Six studies were included in the dose-response analysis9,21,28,29,30,33. There was an 4% reduction in risk for per 2 years delay in menarcheal age (summarized RR = 0.96; 95% CI = 0.94–0.98) with moderate but non-significant heterogeneity (I2 = 45.7%, P = 0.101) (Table 3, Fig. 3). There was no indication of a publication bias by using Egger’s test (P-bias = 0.152) and no asymmetry was observed in funnel plots when inspected visually. There was no evidence of a nonlinear association between the menarcheal age and endometrial cancer risk (P for nonlinearity = 0.16).
Dose-response analysis (random effects model) between per 2 year delay in menarcheal age and risk of endometrial cancer.
Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk estimate with its 95% CI. CI: confidence interval; RR: relative risk.
Subgroup analyses
In subgroup analyses, possible differences between risk estimates by various study characteristics were examined. The finding of decreased endometrial cancer risk with later menarcheal age was consistently observed in almost all of the stratified analyses, although not all strata showed statistical significance. Furthermore, there was no evidence of significant heterogeneity between subgroups detected by meta-regression analyses (Table 2 and Table 3). When stratified by whether considering or adjustment for potential confounders, we did not find a significant difference between estimates adjusted and those not adjusted for specific factors (Table 2). Likewise, similar results were also observed in the dose-response analysis (Table 3).
Sensitivity analyses
In a sensitivity analysis, we sequentially removed one study at a time and re-analyzed the data. The 8 study-specific RRs of the oldest versus youngest menarcheal age ranged from a low of 0.65 (95% CI: 0.53–0.79, I2 = 40.8%, P = 0.119) after omission of the study by Yang et al.28 to a high of 0.74 (95% CI: 0.66–0.83, I2 = 0%, P = 0.461) after omission of the study by Wernli et al.9. Similar analyses were also carried out in the dose-response analysis, with study specific RRs ranged from a low of 0.95 (95% CI: 0.93–0.97, I2 = 15.7%, P = 0.314) after omission of the study by Yang et al.28 to a high of 0.97 (95% CI: 0.96–0.99, I2 = 10.7%, P = 0.345) after omission of the study by Wernli et al.9.
Discussion
To the best of our knowledge, this is the first comprehensive and quantitative assessment to explore the relationship between menarcheal age and risk of endometrial cancer. Overall, menarcheal age was found to be associated with an estimated 32% reduction in endometrial cancer risk when the oldest menarcheal age category compared to the youngest. Additionally, the results of dose-response analyses suggested an 4% reduction in endometrial cancer risk for per 2 years delay in menarcheal age (summarized RR = 0.96, 95% CI: 0.94–0.98). These significant results were consistent within subgroup analyses and across sensitivity analyses.
The association between hormonal risk factors and endometrial cancer risk could be partly attributed to the “unopposed estrogen” hypothesis. This hypothesis proposes that exposure to estrogen, in the absence of sufficient progestin, leads to increase mitotic activity, DNA replication and somatic mutations of endometrial cells that may result in malignant transformations2,3,4,5,34,35. In addition, as the beginning of the menstruation span, later menarcheal age might decrease the risk of endometrial cancer by decreasing a woman’s lifetime number of ovulations which was characterized by exposure to endogenous estrogen and progesterone deficiency. Several epidemiologic studies8,9,20,21,23,35 have reported that longer year of menstruation span significantly increased the risk of endometrial cancer, which partly supported the findings in this meta-analysis, as well as the hypothesis which suggested that endometrial cancer risk might related to the lifetime number of ovulations. A recent study36, the Epidemiology of Endometrial Cancer Consortium (E2C2), has pooled of 15 observational studies (10 prospective and 5 case-control studies), provided evidence that older menarcheal age was significant inverse associated with endometrioid endometrial carcinoma (≥15 versus. <11 menarcheal age, OR: 0.63, 95% CI: 0.54–0.72, P trend = 0.04), which partly supported the findings of this meta-analysis. However, none of the included studies of this meta-analysis demonstrated the information of different histological type which limited us to confirm their finding. On the other hand, Type I endometrial cancers are mostly endometrioid adenocarcinomas, which seem to develop from abnormal glandular proliferations (i.e., endometrial hyperplasia) driven by hormonal mechanisms. In contrast, Type II endometrial cancers often display serous or clear cell histology and arise from atrophic endometrium in a less hormonally dependent manner. In addition, subtypes of these cancers are characterized by distinctive molecular alterations and endometrioid carcinomas are more clearly linked to elevated levels of sex-steroid hormones and expression of hormone receptors28,37,38. However, only Yang et al.28 divided the main analyses by the subtype of endometrial cancer. Given this, further studies are warranted to focus on this issue. In our previous meta-analysis of menarcheal age and ovarian cancer risk11, we found that the categories used for the age at menarche varied from studies. Although the results of meta-regression of our study found no evidence for heterogeneity of different study populations, each population was unique and the category of menarcheal age might different from other studies. For example, Wernli et al.9 reported that the youngest and oldest category of menarcheal age in a cohort study with 267,400 women which was carried out in China was less than 13 years and over than 17 years, respectively. By comparison, Olson et al. reported that the youngest and oldest category of menarcheal age in a cohort study with 24,848 women which was carried out in Iowa was less than 11 years and over than 14 years, respectively. Since this, the interpretation of the results when using the oldest category of menarcheal age compared to the youngest should be cautiously. However, the results of dose-response analysis which could reduce the impact from the different category of menarcheal age of these included studies were in accordance with the results of oldest versus youngest category analysis which not only demonstrated the findings of this meta-analysis were robust but suggested that this aforementioned association was independent of the category methods. On the other hand, several genome-wide association studies (GWAS) have identified that genetic factors may associated with menarcheal age39,40,41,42 which also might attributed to the different category of menarcheal age. Therefore, given six prospective studies were included in the dose-response analysis of this study, more epidemiologic studies should provide the risk estimates which modeling a one or two-year increment in menarcheal age continuously, as well as focus on whether genetic polymorphisms may modify the association between menarcheal age and endometrial cancer risk.
A strength of this study is that it included a relatively larger sample sizes than any other individual studies with a total of 4553 endometrial cancer cases and approximately 949,945 non-cases. Thus, we had statistical power to detect moderate and weak associations and carry out subgroup analyses by potential confounding factors and study characteristics. Another important strength of this meta-analysis was that we included all the prospective design studies and the results are unlikely to be explained by the bias of traditional retrospective studies.
This study, however, also has limitations. First, since all included prospective studies were based on the observational design, it is possible that these aforementioned observed significant inverse associations could be due to the unmeasured or residual confounding. Many factors known to affect endometrial cancer risk, especially BMI12,43 is either associated with menarcheal age or as an important risk factor for endometrial cancer. However, since we could not get the access of raw data of each included studies and none of them have clarified the detail information of potential confounders in their published articles, thus we carried out the stratified analyses by these potential confounders which were adjusted in the multivariable models of their primary analyses. On the other hand, cohort studies more likely to present the age at menarche or other important risk factors of endometrial cancer by the distribution of exposure instead of by case status, which might partly results in limited studies included in this meta-analysis. Although numerous subgroup analyses were carries out and the results generally showed inverse associations, the limitation by the numbers of included prospective studies still should be considered. Second, because of the majority of the prospective study included the participants later than the focused exposure happened, thus females included in this study generally reported their exposure information long after menarche which may result in potential information bias. However, given that menarche is the milestone of puberty initiation and Bean et al.44 previously reported that age at menarche has reasonably good recall accuracy. Therefore, this kind of information bias could be limited in our study. Third, to our knowledge, the category of menarcheal age differed between studies and may have contributed to the heterogeneity in results. However, few of the included studies reported how they categorized the menarcheal age and thus, we hardly considered this point in the subgroup analysis and ruled out the heterogeneity thoroughly. Furthermore, publication bias can also be a problem in meta-analyses of published studies but we found no statistical evidence of publication bias in this meta-analysis. Finally, as the starting point of a woman’s menstrual history, menarcheal age only focused on the early period of menstrual history, future studies should provide more evidence of the reproductive factors of other periods.
In summary, this meta-analysis suggested that later menarcheal age was inversely associated with the risk of endometrial cancer. Further studies, especially large consortium or pooled studies would be of interest to fully adjust for potential confounders and distinguish whether other risk factors which closely associated with both menstruation span and hormone change (e.g., age at menopause and multiparity) might have the similar inverse association with endometrial cancer risk and whether these associations might differ by the histological subtypes of endometrial cancer.
Methods
Literature search
We performed a comprehensive literature search to the end of June 2015 using the MEDLINE (PubMed) and EMBASE databases limited to the studies of humans by using the following search key words and Medical Subject Headings terms: (menarche OR reproductive factors OR reproduction) AND (endometrial OR endometrium OR corpus uteri OR uterine corpus) AND (cancer OR neoplasm OR carcinoma OR tumor). Additionally, the reference lists of retrieved articles were also scrutinized for additional studies by manual search. A similar search strategy was utilized for our previous meta-analysis of menarcheal age and ovarian cancer11. This study was planned, conducted and reported in adherence to standards of quality for reporting meta-analyses45.
Study selection criteria
Published studies were included by the following selection criteria if they 1) used a prospective study design, including cohort, case-cohort and nested case-control studies; 2) evaluated the association between menarcheal age and incident endometrial cancer risk; and 3) presented relative risk (RR) or hazard ratio (HR) estimates with 95% confidence intervals (CI), standard errors (SE) or data necessary to calculate these.
Published manuscripts were excluded by the following exclusion criteria if they 1) had a retrospective design; 2) the estimates were presented without SE or other information that allowed calculation of SE; 3) reported exclusively on endometrial cancer mortality. When multiple publications from the same study were available, we used the publication with the largest number of cases and most applicable information.
All above study selection and exclusion procedures were carried out by two independent investigators (T-TG and X-XM).
Data abstraction and quality assessment
For each eligible study, two investigators (T-TG and X-XM) independently performed the eligibility evaluation and data abstraction. Discrepancies were settled by consensus or by involving a third reviewer (Y-LW) for adjudication. Data abstracted from each study were: author list, year of publication, country where the study was performed, study design, study sample size (number of cases and cohort size), range of follow-up for cohort studies, exposure and outcome assessment and menarcheal age categories, study-specific adjusted RRs or HRs with their 95% CIs for the oldest versus youngest category of menarcheal age (we extracted the RRs that reflected the greatest degree of control for potential confounders for use in the main analyses) and factors matched between cases and controls in nested case-control study and potential confounders adjusted in the data analysis.
Since all included studies are prospective study and similar to our previous studies11,46, we did not assign quality scores47 to assess the methodological quality of all the included studies which lacks demonstrated validity and sometimes results may not be associated with quality48, but investigated whether specific study characteristics, such as study design and adjustment for confounders, which are indicators of study quality, influenced the results in subgroup analyses.
Statistical analysis
The study-specific adjusted RRs were used as the common measure of association across studies. For the meta-analysis, we assumed that estimates of risk, rate or hazard ratios from prospective studies were all valid estimates of the RR and we therefore report all results as the RR for simplicity. For study of Yang et al.28 that reported results separately of type I and type II endometrial cancer, but not combined, we pooled the results using a fixed-effect model to obtain an overall combined estimate before combining with the rest of the studies.
The possible heterogeneity in results across studies was examined by using the I2 statistics49. The I2 statistic represents the proportion of total variation contributed by between-study variation49. We used a random-effects model to calculate summary RR of endometrial cancer with 95% CIs, considering within- and between-study variation50. Heterogeneity between subgroups was evaluated by meta-regression46.
For the dose-response analysis, we used the generalized least-squares trend estimation method developed by Greenland et al.51 and Orsini et al.52 to compute study-specific slopes (linear trends) and 95% CIs from the natural logs of the RRs and CIs across categories of the menarcheal age. The aforementioned method requires the following information: 1) the distribution of cases and person-years or non-cases and the RRs with the variance estimates for at least three quantitative exposure categories; 2) the median or mean level of these exposures in each category (if reported by ranges, mean level were calculated by averaging the lower and upper bound; if the lowest category was open ended, the lowest boundary was considered to be zero; if the highest category was open ended, the open-ended interval length was assumed to be the same as the adjacent interval). Since these criteria, six studies9,21,28,29,30,33 were included in the dose-response analysis of menarcheal age and endometrial cancer risk. Furthermore, a potential nonlinear dose-response relationship between the menarcheal age and endometrial cancer risk was modeled by using restricted cubic splines with 3 knots at fixed percentiles (10%, 50% and 90%) of the distribution of exposure. We calculated an overall P value by testing that these two regression coefficients were simultaneously equal to zero. We calculated a P value for nonlinearity by testing that the coefficient of the second spline was equal to zero. The details of this method has been published elsewhere53,54. The dose-response results are presented for a two years delay in menarcheal age.
To investigate possible sources of heterogeneity of main results, we carried out stratified analyses by the following study features: duration of follow-up (<10 versus ≥10 years), number of cases (<400 versus ≥400), method of exposure assessment (self-administered questionnaire versus trained interviewers), study population (American versus Non-American) and factors matched between cases and controls in nested case-control study and potential confounders adjusted in the data analyses (body mass index, parity, oral contraceptive use, exogenous hormones use, menopause status and smoking status). Finally, sensitivity analysis was executed by deleting each study in turn to reflect the influence of individual data set on the overall estimate.
Small study bias, such as publication bias, was evaluated by Egger’s linear regression55 and funnel plots. A P-value less than 0.05 for Egger’s test was considered representative of significant statistical publication bias. Statistical analyses were performed with Stata (version 11.2; StataCorp, College Station, TX). P-values were two sided with a significance level of 0.05.
Additional Information
How to cite this article: Gong, T.-T. et al. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci. Rep. 5, 14051; doi: 10.1038/srep14051 (2015).
References
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011).
Cook, L. S., Weiss, N. S., Doherty, J. A. & Chen, C. Endometrial Cancer. In: Cancer epidemiology and prevention. 3rd edn, (eds Schottenfeld, D. & Fraumeni, Jr. ) 1027–1043. (Oxford University Press, 2006).
Key, T. J. & Pike, M. C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 57, 205–12 (1988).
Ruddon, R. W. Cancer Biology, 3rd edn, 240–251. (Oxford University Press, 1995).
Pitot, H. C. Fundamentals of Oncology. (Marcel Dekker Inc, 1986).
King, R. J. B. & Whitehead, M. I. Estrogen and progestin effects on epithelium and stroma from pre- and postmenopausal endometria: application to clinical studies of the climacteric syndrome. In: Steroids and Endometrial Cancer. (eds Jasonni, V. M. ) (New York Raven Press, 1983).
Akhmedkhanov, A., Zeleniuch-Jacquotte, A. & Toniolo, P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann N Y Acad Sci 943, 296–315 (2001).
Pettersson, B., Adami, H. O., Bergstrom, R. & Johansson, E. D. Menstruation span—a time-limited risk factor for endometrial carcinoma. Acta Obstet Gynecol Scand 65, 247–55 (1986).
Wernli, K. J. et al. Menstrual and reproductive factors in relation to risk of endometrial cancer in Chinese women. Cancer Causes Control 17, 949–55 (2006).
Li, C. Y. et al. Age at menarche and risk of colorectal cancer: a meta-analysis. PLoS One 8, e65645 (2013).
Gong, T. T. et al. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer 132, 2894–900 (2013).
Currie, C. et al. Is obesity at individual and national level associated with lower age at menarche? Evidence from 34 countries in the Health Behaviour in School-aged Children Study. J Adolesc Health 50, 621–6 (2012).
Bralic, I. et al. Association of early menarche age and overweight/obesity. J Pediatr Endocrinol Metab 25, 57–62 (2012).
Mandel, D. et al. Age at menarche and body mass index: a population study. J Pediatr Endocrinol Metab 17, 1507–10 (2004).
Jacobsen, B. K., Heuch, I. & Kvale, G. Association of low age at menarche with increased all-cause mortality: a 37-year follow-up of 61,319 Norwegian women. Am J Epidemiol 166, 1431–7 (2007).
Tamakoshi, K., Yatsuya, H. & Tamakoshi, A. Early age at menarche associated with increased all-cause mortality. Eur J Epidemiol 26, 771–8 (2011).
Wu, X. et al. Age at menarche and natural menopause and number of reproductive years in association with mortality: results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One 9, e103673 (2014).
Mueller, N. T. et al. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: the Singapore Chinese Health Study. Ann Epidemiol 22, 717–22 (2012).
Brinton, L. A. et al. Reproductive, menstrual and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol 167, 1317–25 (1992).
Xu, W. H. et al. Menstrual and reproductive factors and endometrial cancer risk: Results from a population-based case-control study in urban Shanghai. Int J Cancer 108, 613–9 (2004).
Dossus, L. et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 127, 442–51 (2010).
Wernli, K. J. et al. Occupational risk factors for endometrial cancer among textile workers in Shanghai, China. Am J Ind Med 51, 673–9 (2008).
McPherson, C. P. et al. Reproductive factors and risk of endometrial cancer. The Iowa Women’s Health Study. Am J Epidemiol 143, 1195–202 (1996).
Kabat, G. C. et al. Adult height in relation to risk of cancer in a cohort of Canadian women. Int J Cancer 132, 1125–32 (2013).
Khan, M. et al. Risk of endometrial cancer mortality by ever-use of sex hormones and other factors in Japan. Asian Pac J Cancer Prev 7, 260–6 (2006).
Kasum, C. M. et al. Whole grain intake and incident endometrial cancer: the Iowa Women’s Health Study. Nutr Cancer 39, 180–6 (2001).
Bergkvist, L., Persson, I., Adami, H. O. & Schairer, C. Risk factors for breast and endometrial cancer in a cohort of women treated with menopausal oestrogens. Int J Epidemiol 17, 732–7 (1988).
Yang, H. P. et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol 177, 142–51 (2013).
Karageorgi, S., Hankinson, S. E., Kraft, P. & De Vivo, I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses’ Health Study cohort 1976–2004. Int J Cancer 126, 208–16 (2010).
Olson, J. E., Sellers T. A., Anderson K. E. & Folsom, A. R. Does a family history of cancer increase the risk for postmenopausal endometrial carcinoma? A prospective cohort study and a nested case-control family study of older women. Cancer 85, 2444–9 (1999).
Setiawan, V. W. et al. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol 165, 262–70 (2007).
Kvale, G., Heuch, I. & Ursin, G. Reproductive factors and risk of cancer of the uterine corpus: a prospective study. Cancer Res 48, 6217–21 (1988).
Hisada, M. et al. Prospective study of antibody to human papilloma virus type 16 and risk of cervical, endometrial and ovarian cancers (United States). Cancer Causes Control 12, 335–41 (2001).
Parazzini, F., La Vecchia, C., Bocciolone, L. & Franceschi, S. The epidemiology of endometrial cancer. Gynecol Oncol 41, 1–16 (1991).
Zucchetto, A. et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev 18, 316–21 (2009).
Felix, A. S. et al. The etiology of uterine sarcomas: a pooled analysis of the epidemiology of endometrial cancer consortium. Br J Cancer 108, 727–34 (2013).
Sherman, M. E. et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol 10, 963–8 (1997).
Lax, S. F., Pizer, E. S., Ronnett, B. M. & Kurman, R. J. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen and progesterone receptor expression. Hum Pathol 29, 551–8 (1998).
He, C. et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 41, 724–8 (2009).
Ong, K. K. et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet 41, 729–33 (2009).
Liu, Y. Z. et al. Genome-wide association analyses identify SPOCK as a key novel gene underlying age at menarche. PLoS Genet 5, e1000420 (2009).
Sulem, P. et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet 41, 734–8 (2009).
Crosbie, E. J. et al. Body mass index, hormone replacement therapy and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 19, 3119–30 (2010).
Bean, J. A. et al. Variations in the reporting of menstrual histories. Am J Epidemiol 109, 181–5 (1979).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–12 (2000).
Wu, Q. J. et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 24, 1079–87 (2013).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Date of access: 10/March/2015).
Greenland, S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 140, 290–6 (1994).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986).
Greenland, S. & Longnecker, M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–9 (1992).
Orsini, N. et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations and software. Am J Epidemiol 175, 66–73 (2012).
Royston, P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 19, 1831–47 (2000).
Bagnardi, V., Zambon, A., Quatto, P. & Corrao, G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 159, 1077–86 (2004).
Egger, M., Davey, S. G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997).
Acknowledgements
This work was supported by The National Natural Science Foundation of China (81472438 and 81272874 for Xiao-Xin Ma). We would like to thank Dr. Qi-Jun Wu (Department of Clinical Epidemiology, Shengjing Hospital of China Medical University) for assistance in the interpretation of the results.
Author information
Authors and Affiliations
Contributions
T.-T.G., Y.-L.W. and X.-X.M. designed research; T.-T.G., Y.-L.W. and X.-X.M. conducted research; T.-T.G. and X.-X.M. analyzed data; T.-T.G., Y.-L.W. and X.-X.M. wrote the draft; All authors read, reviewed and approved the final manuscript. X.-X.M. had primary responsibility for final content.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gong, TT., Wang, YL. & Ma, XX. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci Rep 5, 14051 (2015). https://doi.org/10.1038/srep14051
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14051
This article is cited by
-
The J shaped association of age at menarche and cardiovascular events: systematic review and meta-analysis
Scientific Reports (2024)
-
Analysis of transcriptional changes in the immune system associated with pubertal development in a longitudinal cohort of children with asthma
Nature Communications (2023)
-
Risk factors for endometrial cancer in Black women
Cancer Causes & Control (2023)
-
Occupational exposure to pesticides and endometrial cancer in the Screenwide case-control study
Environmental Health (2023)
-
Racial/ethnic differences in anthropometric and hormone-related factors and endometrial cancer risk: the Multiethnic Cohort Study
British Journal of Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.