Abstract
Excess aldosterone secretion in patients with primary aldosteronism (PA) impairs their cardiovascular system. Heart rhythm complexity analysis, derived from heart rate variability (HRV), is a powerful tool to quantify the complex regulatory dynamics of human physiology. We prospectively analyzed 20 patients with aldosterone producing adenoma (APA) that underwent adrenalectomy and 25 patients with essential hypertension (EH). The heart rate data were analyzed by conventional HRV and heart rhythm complexity analysis including detrended fluctuation analysis (DFA) and multiscale entropy (MSE). We found APA patients had significantly decreased DFAα2 on DFA analysis and decreased area 1–5, area 6–15 and area 6–20 on MSE analysis (all p < 0.05). Area 1–5, area 6–15, area 6–20 in the MSE study correlated significantly with log-transformed renin activity and log-transformed aldosterone-renin ratio (all p < = 0.01). The conventional HRV parameters were comparable between PA and EH patients. After adrenalectomy, all the altered DFA and MSE parameters improved significantly (all p < 0.05). The conventional HRV parameters did not change. Our result suggested that heart rhythm complexity is impaired in APA patients and this is at least partially reversed by adrenalectomy.
Similar content being viewed by others
Introduction
Primary aldosteronism (PA) is characterized by an inappropriate production of aldosterone and affects 5–13% of hypertensive patients1. Recent studies indicate that PA is as the most frequent cause of secondary hypertension1.
Patients with PA have a higher incidence of cardiovascular events than patients with essential hypertension (EH) matched for hypertension severity2. PA patients also have a higher incidence of altered cardiac structure, such as left ventricular hypertrophy and cardiac fibrosis than EH patients, which may due to the high plasma aldosterone levels in these patients3. As well as altering cardiac structure, excess aldosterone also increases vascular stiffiness4. After removal of aldosterone excess by adrenalectomy or blockade of aldosterone action by mineralocorticoid receptor antagonists, the altered structure of the cardiovascular system recover4,5.
Aldosterone also influences the autonomic system. In animal studies, infusion of aldosterone into cerebral ventricles increases sympathetic nerve activity (SNA) and blood pressure (BP), effects which are antagonized by mineralocorticoid antagonist administration6 . In clinical studies, the reported effects of aldosterone excess on the autonomic system have been inconsistent. Three muscle SNA studies were measured by intraneural microelectrodes in PA patients gave conflicting results7,8,9.
Analysis of the variation of heart rate oscillation, mostly known as heart rate variability (HRV), is commonly used to assess the alteration of autonomic function in human studies due to its simple, noninvasive and inexpensive approach10. Traditional linear analysis of HRV is also used as a tool to evaluate the autonomic system and especially high frequency power spectrum analysis to evaluate parasympathetic function10. HRV has been commonly applied to predict outcome in patients with cardiovascular disease11. In recent years, newer methods based on nonlinear and nonstationary signal modeling have been developed and successfully applied12. The concept of heart rhythm complexity analysis by nonlinear methods including detrended fractal analysis (DFA) or multiscale entropy (MSE) is based on the assumption that a healthy system exhibits a meaningful complex control over different time scales to maintain operation in ever-changing environment13,14. Conversely, decreased complexity of heart rate dynamics has been demonstrated in patients with diseases states, such as heart failure, stroke, sepsis and critical illness requiring extracorporeal life support15,16,17,18. Compared to traditional HRV parameter based on linear methodology, heart rhythm complexity analysis showed better predictive power for prognosis in patients with cardiovascular disease15,19.
Whether aldosterone excess affects heart rhythm complexity is unclear and formed the basis of the current study.
Results
Patients
Twenty patients (9 men) with aldosterone producing adenoma (APA) undergoing adrenalectomy and 25 patients with EH were enrolled. The clinical data are shown in Table 1. Patients with APA had significantly higher plasma aldosterone concentration (PAC), higher left ventricular mass index, higher plasma aldosterone-to-renin activity ratio (ARR), lower serum potassium levels and lower plasma renin activity (PRA) than patients with EH. Regarding medication usage, a significantly higher percentage of APA patients received α-blocker and spironolactone treatment and lower percentage received angiotensin receptor blocker (ARB) treatment.
Holter data
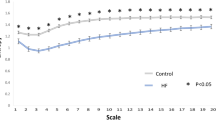
For all (Table 2) linear Holter parameters, there were no differences between the APA and EH groups (all p > 0.05). For non-linear parameters, the APA group had significantly higher DFAα2 (1.19 ± 0.09 vs. 1.13 ± 0.10, p = 0.046), lower area 1–5 (5.4 ± 1.3 v.s. 6.4 ± 1.0, p = 0.006), lower area 6–15 (13.4 ± 2.4 vs. 14.9 ± 1.8, p = 0.023) and lower area 6–20 (20.4 ± 3.6 vs. 22.4 ± 2.7, p = 0.036) of the MSE parameters than EH group. The entropy over different time scales in APA and EH groups was shown in Fig. 1a. The MSE parameters, including area 1–5, area 6–16 and area 6–20 significantly correlated with Log PRA and Log ARR (all p < 0.01) (Table 3). Serum potassium levels also significantly correlated with area 6–16 and area 6–20 (both p < 0.05).
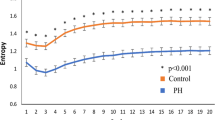
The entropy over different time scales.
a) The square open symbols represented the entropy of patients with EH and the black solid dots the entropy of APA patients before surgery. b) The black solid dots represented the entropy of APA patients before surgery and the open circles the entropy of APA patients after surgery. *p < 0.05.
In multi-variate regression analysis, Log PRA was an independent factor associated with area 6–15 (β = 0.803, p = 0.007) and area 6–20 (β = 1.158, p = 0.010) in the final models (excluded variables: serum potassium levels and Log ARR in both models).
Post-adrenalectomy follow-up
By one year after adrenalectomy, the number of anti-hypertensive medications, log PAC, log ARR and left ventricular mass index had significantly decreased while PRA and serum K level had increased (Table 4). Eleven out of 20 patients were cured of hypertension.
Repeat Holter study (Table 5) did not reveal any significant change in linear HRV parameters post-operatively. In contrast, DFAα2 decreased significantly (from 1.19 ± 0.09 to 1.13 ± 0.13, p = 0.043). The entropy over different time scales in pre- and post-operative APA patients was shown in Fig. 1b. Within the MSE parameters, slope 5 (from 0.067 ± 0.055 to 0.098 ± 0.051, p = 0.035), area 1–5 (from 5.4 ± 1.3 to 6.3 ± 1.1, p = 0.007), area 6–15 (from 13.4 ± 2.4 to 15.5 ± 1.8, p = 0.001) and area 6–20 (from 20.4 ± 3.6 to 23.4 ± 2.7, p = 0.003) increased significantly.
Discussion
The major findings of this study were 1) APA patients had worse heart rhythm complexity than EH patients and this was independent of BP; 2) In the correlation study regarding all participants, the parameters of heart rhythm complexity correlated with Log PRA and Log ARR, but not blood pressure or altered cardiac structure; 3) the impaired heart rhythm complexity improved after adrenalectomy.
The presented study is the first study to show the adverse effects of aldosterone on heart rhythm complexity, which quantifies the complex regulatory dynamics of human physiology20. HRV is commonly used to assess autonomic function and risk stratification of patients with cardiovascular disease11,21. Compared to traditional linear HRV parameters, nonlinear metrics (including DFA and MSE) showed better predictive power for clinical outcomes in heart failure patients and in an experimental sepsis model15,19,22. Recently, the MSE method, specifically developed to treat heterogeneous complexity, was shown to extend the traditional entropy algorithm to quantify the information richness over multiple time scales in physiological systems20. This complex structure will “break down” in diseased patients, such as those with heart failure or with critical illness and may be further affected in those with poor prognosis15,18. In our previous study, MSE provided the best prognostic prediction in patients with heart failure15. MSE also predicted outcome of severe traumatic patients requiring intensive care unit admission across the diverse spectrum of traumatic injury23, the neurological outcome after stroke16, the clinical consequences of sepsis17 and outcome of patients with critical illness receiving extracorporeal life support18. In the present study, PA patients had worse heart rhythm complexity than EH patients, suggesting that aldosterone excess impaired the complex regulatory dynamics of human physiology. Furthermore, there were significant associations between heart rhythm complexity and log PRA and log ARR, but not BP or cardiac structure in the correlation study regarding all participants. This implied a direct association between aldosterone and heart rhythm complexity. Moreover, after adrenalectomy, the impaired heart rhythm complexity improved, suggesting that the impairment is at least partially reversible.
In contrast to the significant differences of values in non-linear parameters between PA and EH patients, the values of traditional HRV parameters (such as time and frequency domain parameters) were comparable in both groups in the current study. This also applied when comparing the pre and post-operative data of APA patients. As mentioned earlier, traditional HRV parameters are commonly used to evaluate the autonomic system and particularly high frequency power spectrum analysis to evaluate parasympathetic function10. Data from the current study did not provide further evidence to support PA patients having worse autonomic function than EH patients.
The relation between aldosterone and the autonomic system is complex. In animal studies, excess of aldosterone is associated with autonomic dysfunction. It was found that there are mineralocorticoid receptors near the hippocampus and regulated by mineralocorticoids and glucocorticoids24. Further, aldosterone infusion into cerebral ventricles increased SNA and BP, an effect which was antagonized by administration of a mineralocorticoid antagonist6. In another animal model, the increased BP induced by aldosterone was blocked by chemical sympathectomy25. In the elderly hypertensive population, the mineralocorticoid antagonist spironolactone decreased norepinephrine levels26. The evidence suggests a close relationship between aldosterone and the autonomic system.
Whether PA patients have impaired autonomic function is controversial. Acute infusion of aldosterone in healthy volunteers increased the standard deviation of RR intervals and total power and was associated with a trend towards increased time domain HRV parameters27. However, in that study, basal muscle SNA, BP and heart rate remained unaffected by aldosterone administration. This suggests that, acutely, aldosterone infusion tends to increase cardiac vagal activity and has no effect on sympathetic activity. PA patients have lower sympathetic vasomotor tone than patients with EH28. Several studies have evaluated muscle SNA in patients with PA7,8. In the study by Kontak et al., PA patients had similar muscle SNA to EH patients7. Both PA and EH patients had higher muscle SNA than normotensive subjects. However, the muscle SNA decreased significantly after adrenalectomy, accompanied by a decrease in BP7. Whether removal of aldosterone excess or decrease of BP contributed to this improvement is unclear. In another study by Miyajima et al., PA patients had lower muscle SNA compared to EH patients8. In a third study by Matsukawa et al.9, PA patients have lower muscle SNA compared to normotensive subjects. Interestingly, the muscle SNA was significantly elevated after unilateral adrenalectomy in PA patients. It was in contrast to the study by Kontak et al.7. Although different race and other inter-individual differences may have contributed to this disparity, it may also reflect the fact that regulation of sympathetic activity in PA patients is complex and influenced by factors other than aldosterone itself.
The relationship between aldosterone and parasympathetic activity is also complex and conflicting data exist. Acute infusion of aldosterone in healthy volunteers increased cardiac vagal activity in one HRV study27. Aliskirin, which reduces activity of the renin-angiotensin-aldosterone system via direct inhibition of renin, increased parasympathetic function as measured by two cardiovascular autonomic reflex tests29. However, the high frequency domain of HRV, an index of cardiac parasympathetic tone did not change29. Studies assessing parasympathetic function in PA are quite few. To the best of our knowledge, there are only two studies from the same group dealing with this issue. In the first, the baroreflex sensitivity of PA patients was found to be similar to age-matched healthy subjects. In contrast, the baroreflex sensitivity was impaired in EH patients30. In the second study from the same group, PA patients showed similar high frequency in HRV compared to normotensive subjects, but high frequency in HRV was decrease in patients with EH28. These data suggest that, although hypertensive, PA patients have intact parasympathetic activity. Against this notion, however, was the observation that the high frequency value decreased after adrenalectomy in the PA patients28.
In this study, we only enrolled APA patients that underwent adrenalectomy, but not patients with bilateral adrenal hyperplasia. Adrenalectomy is the treatment of choice for APA patients. In contrast, medical treatment with spironolactone is the treatment of choice in patients with bilateral adrenal hyperplasia. Compared to medical treatment with spironolactone, adrenalectomy decreases left ventricular mass more quickly31. In another study, only adrenalectomy but not spironolactone improved arterial stiffness in PA patients (average follow-up period: one year)32. This evidence implies that adrenalectomy is a more effective method to reverse the effects of aldosterone on cardiovascular system. Therefore, we enrolled APA patients that underwent adrenalectomy as the study group to enhance the difference between pre- and post-treatment.
One advantage of this study is that the BP and the number of antihypertensive medications was comparable between APA and EH groups. It is common that PA patients have significantly higher BP than EH patients33. In our previous studies, APA patients also had significantly higher BP than EH patients4,5. However, any BP difference (for example, if APA patients had significantly higher BP than EH patients in this study) would make the interpretation of results more difficult and complex. Although the correlations between HRV parameters and BP were not significant, we still could not exclude a possible confounding effect from a difference in BP. Thankfully, the BP difference was not significant between APA and EH groups in this study, which made the interpretation easier.
Our study has several limitations. First, the study population is small. Further large prospective studies are needed to confirm the results. Second, we did not measure muscle SNA to evaluate sympathetic activity. However, the main purpose of this study was to evaluate the influence of aldosterone on heart rhythm complexity rather than sympathetic activity per se.
In conclusion, APA patients had impaired heart rhythm complexity compared to EH patients and this was independent of BP. The impaired heart rhythm complexity improved after adrenalectomy.
Methods
Patients
This prospective study enrolled 20 patients diagnosed with unilateral APA and who underwent adrenalectomy during the period from December 2006 to October 2009. The patients were evaluated and registered in the Taiwan Primary Aldosteronism Investigation (TAIPAI) database. The database was constructed for quality assurance in two medical centers (National Taiwan University Hospital (NTUH), Taipei; Taipei Medical University Hospital, Taipei), three metropolitan hospitals (Cardinal Tien Hospital, New Taipei City; Taipei Tzu Chi Hospital, New Taipei City; Yun-Lin Branch of NTUH, Douliou City) and two local hospitals (Hsin-Chu Branch of NTUH, Hsin-Chu City; Zhongxing Branch of Taipei City Hospital, Taipei)34,35,36. In addition, another 25 patients with EH were enrolled as the control group. Medical history including demography and medication was carefully recorded. Biochemical parameters were measured at the first evaluation of these patients in National Taiwan University Hospital. PRA was measured as the generation of angiotensin-I in vitro using a commercially available radioimmunoassay kit (Cisbio, Bedford, MA); PAC was measured by radioimmunoassay with commercial kits (Aldosterone Maia Kit; Adaltis Italia, Bologna, Italy). All antihypertensive medications were discontinued for at least 21 days before measuring plasma PRA and PAC levels. Diltiazem and/or doxazosin were administered for control of marked high blood pressure when required. All APA patients underwent 24-h ambulatory ECG Holter recording (MyECG E3-80, Mircostar Company, Taipei) within 3 months before operation and one year after operation. EH patients also underwent 24-h ambulatory ECG Holter recording at the time of enrollment. The ECG signals were sampled at 250 Hz and stored in secure digital memory card for offline analysis on a microcomputer. This study was approved by the Institutional Review Board of National Taiwan University Hospital and all subjects gave informed consent in written form including for the storage of their information in the hospital database and usage for research. The methods in the study were carried out in accordance with the approved guidelines.
Data Pre-Processing
Each digitalized 24-hour ECG data was annotated by an automatic algorithm then carefully inspected and corrected by the technicians for extracting the RR intervals and the ectopic beats were interpolated by its adjacent RR intervals. A four-hour length of RR intervals within daytime (between 9 AM–5 PM) was selected for analysis in order to avoid the confounding effects that may occur due to sleep or diurnal rhythm37. Only the RR series of subjects in whom qualified normal sinus beats made up more than 80% of the recording were included for further analysis.
Time and frequency domain analysis
The standard deviation of normal RR intervals, the percentage of the absolute change in consecutive normal RR interval exceeds 50 ms and percentage of the absolute change in consecutive normal RR interval exceeds 20 ms were calculated to represent the total variance and vagal modulation of heart rate. The spectrum analyses were carried out according to the recommendations from the European Society of Cardiology and the North American Society of Pacing Electrophysiology10. Instead of calculating the spectrum in overall length, we divided the data into 16 segments and Fourier transformation was performed individually to avoid the influence of external nonstationarity. The spectral density of each frequency band- high frequency (0.15–0.4 Hz), low frequency (0.04–0.15 Hz) and very low frequency (0.003–0.04 Hz) were computed by averaging the absolute powers (msec2) in separated segments.
Nonlinear methods
Nonlinear analysis enables researchers to probe the fundamental characteristics of the signals. We apply two methods (DFA and MSE) for their ability to evaluate the underling properties of the signals hidden beneath the seemingly chaotic dynamics20,38.
DFA analysis
DFA is a modified root-mean-square analysis that is used to evaluate the fractal correlation (a time-invariant property) beneath the heart rate fluctuation originated from the well-interacted regulatory mechanisms38. First, it eliminates the environmental inferences by removing the linear-fitted “local” trend over different time scales in an integrated time series. Then, the root-mean-square fluctuation of this integrated and detrended time series is calculated. This procedure is repeated in different time scales and then the slope of the curve (α exponent) can be computed on the log-log plot of fluctuation versus box size which indicates the fractal correlation property of the time series.
Since the heart rate oscillation over short timescale is predominated by respiratory sinus arrhythmia, a crossover phenomenon of the α exponent in heart rate dynamics between short (4–11 beats) and long (11–64 beats) time scales has been proposed to provide better understanding of the fractal correlation property in a physiological system38. The short-term (α1) as well as long-term (α2) fractal correlation were calculated.
MSE analysis
In contrast to simply using a single time scale to estimate the predictability of a time series, MSE assesses the complex structure of the physiological signals in different time scales. It comprises two steps: 1) coarse-graining the signals into different time scales; 2) quantifying the degree of predictability in each coarse-grained time series by using sample entropy39. The calculated entropy can be represented as a function of scale provides the meaningful information richness embedded in different time scales. It has been shown that different features of small and large scales in different groups of subjects may assist the clinical categorization20. Therefore, four different parameters were calculated from the MSE profile: the summations of entropy values of scale 1–5 (area 1–5), scale 6–15 (area 6–15), or scale 6–20 (area 6–20) which quantify the complexity exhibited in short and long time scales, respectively; and the linear-fitted slope of scale 1–5 (slope 5) was also calculated to characterize the behavior of short-scale (Fig. 2)20.
Quantification of MSE: The summation of the entropy over different scales can quantify the complexity over certain time scales.
Four parameters of the MSE were assessed. The first two of these were the linear-fitted slope between scales 1–5 (slope 5); the area under curve between scale 1–5 (area 1–5) to represent complexity between short scales. For longer scales, the common profile of entropy gradually increases as the time scale elongates and reaches a plateau around scale 15 where information richness can be accumulated rapidly if the system can respond well. We therefore calculated both the area under curve between scales 6–15 (area 6–15) and 6–20 (area 6–20) to represent complexity between long scales.
In order to avoid an unwanted effect of external nonstationarity which may compromise the entropy-based analysis, we use the empirical mode decomposition method which is based on Hilbert-Huang transform to detrend the original R-R interval signals40. The data was subsequently evaluated by the MSE analysis after processing. Instead of removing trend with a priori mathematical formulas such as polynomial or linear functions, the empirical mode decomposition algorithm could better approximate the hidden trend in complex time series40,41,42.
Diagnosis of APA
APA was diagnosed on the basis on the following four conditions: (1) autonomous excess aldosterone production evidenced with an ARR > 35, a TAIPAI score larger than 60%43 and post-saline loading PAC > 10 ng/dl; (2) adenoma evidenced with a computed tomography (CT) scan on pre-operative evaluation; (3) lateralization of aldosterone secretion at adrenal venous sampling or during dexamethasone suppression NP-59 SPECT/CT44; (4) pathologically proven adenoma after adrenalectomy for those undergoing surgery and subsequent cure or improvement of hypertension control with correction of hypokalemia and normalization of PAC and PRA34,45. Dexamethasone suppression NP-59 SPECT/CT was used in patients who were at risk of contrast nephropathy, had inconclusive results from adrenal venous sampling or had discordant lateralization between imaging studies (such as CT) and adrenal venous sampling.
Echocardiography
A Hewlett-Packard Sonos 5500 ultrasound system equipped with a S3 transducer was used. Echocardiography including two-dimensional, M-mode and Doppler ultrasound recordings were performed. Left ventricular dimension, interventricular septum and posterior wall thicknesses and left ventricular ejection fraction (M-mode) were measured via a parasternal long axis view. Left ventricular mass index was calculated according to the method of Devereux et al.46.
Post-adrenalectomy follow-up
Repeat serum biochemistry, PAC, PRA, 24-h ambulatory ECG Holter recording and echocardiography were performed one year after adrenalectomy. Hypertension was considered cured if the blood pressure decreased to 140/90 mm Hg or less after adrenalectomy and anti-hypertensive medications were not required. These criteria had to be met one year post adrenalectomy31. Patients who were cured within one year but later developed hypertension were still classified as cured.
Statistical analysis
Data were expressed as mean ± SD. Comparisons for continuous data between APA and EH patients were made using the t test. Differences between proportions were assessed by chi-square or Fisher exact test. Comparisons between pre-operative and post-operative parameters were made using paired t test. The Pearson’s correlation test was used to analyze the association among heart rhythm complexity parameters and its determinants. Data of PAC, PRA and ARR were log-transformed before the correlation study due to non-normality which was determined by the Kolmogorov-Smirnov test. Before further analysis, the log-transformed data were tested again to assure the normality of distribution. Significant determinants in the Pearson’s correlation test (p < 0.05) were then tested by a multivariate linear regression test with stepwise subset selection to identify independent factors to predict MSE parameters. A value of p < 0.05 was considered to indicate statistical significance.
Additional Information
How to cite this article: Lin, Y.-H. et al. Reversible heart rhythm complexity impairment in patients with primary aldosteronism. Sci. Rep. 5, 11249; doi: 10.1038/srep11249 (2015).
References
Rossi, G. P. Prevalence and diagnosis of primary aldosteronism. Current hypertension reports 12, 342–348 (2010).
Milliez, P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45, 1243–1248 (2005).
Rossi, G. P. et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 40, 23–27 (2002).
Lin, Y. H. et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 221, 154–159 (2012).
Lin, Y. H. et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J Hypertens 30, 1606–1613 (2012).
Gomez-Sanchez, E. P. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118, 819–823 (1986).
Kontak, A. C. et al. Reversible sympathetic overactivity in hypertensive patients with primary aldosteronism. The Journal of clinical endocrinology and metabolism 95, 4756–4761 (2010).
Miyajima, E. et al. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17, 1057–1062 (1991).
Matsukawa, T. & Miyamoto, T. Does infusion of ANG II increase muscle sympathetic nerve activity in patients with primary aldosteronism? American journal of physiology. Regulatory, integrative and comparative physiology 294, R1873–1879 (2008).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Tsuji, H. et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855 (1996).
Seely, A. J. & Macklem, P. T. Complex systems and the technology of variability analysis. Critical care 8, R367–384, 10.1186/cc2948 (2004).
Yuan, H. K. et al. Acute increase of complexity in the neurocardiovascular dynamics following carotid stenting. Acta neurologica Scandinavica 123, 187–192 (2011).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of complex physiologic time series. Physical review letters 89, 068102 (2002).
Ho, Y. L., Lin, C., Lin, Y. H. & Lo, M. T. The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One 6, e18699 (2011).
Tang, S. C. et al. Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke. Journal of neurology, neurosurgery and psychiatry 86, 95–100 (2015).
Ahmad, S. et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 4, e6642 (2009).
Lin, Y. H. et al. Multi-scale symbolic entropy analysis provides prognostic prediction in patients receiving extracorporeal life support. Critical care 18, 548 (2014).
Makikallio, T. H. et al. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. The American journal of cardiology 87, 178–182 (2001).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of biological signals. Physical review. E, Statistical, nonlinear and soft matter physics 71, 021906 (2005).
La Rovere, M. T. et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107, 565–570 (2003).
Vandendriessche, B. et al. A multiscale entropy-based tool for scoring severity of systemic inflammation. Critical care medicine 42, e560–569 (2014).
Riordan, W. P., Jr., Norris, P. R., Jenkins, J. M. & Morris, J. A., Jr. Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. The Journal of surgical research 156, 283–289 (2009).
de Kloet, E. R. et al. Brain mineralocorticoid receptors and centrally regulated functions. Kidney international 57, 1329–1336 (2000).
Thomas, G. D., O’Hagan, K. P. & Zambraski, E. J. Chemical sympathectomy alters the development of hypertension in miniature swine. Hypertension 17, 357–362 (1991).
Wray, D. W. & Supiano, M. A. Impact of aldosterone receptor blockade compared with thiazide therapy on sympathetic nervous system function in geriatric hypertension. Hypertension 55, 1217–1223 (2010).
Heindl, S. et al. Acute effects of aldosterone on the autonomic nervous system and the baroreflex function in healthy humans. Journal of neuroendocrinology 18, 115–121, 10.1111/j.1365-2826.2005.01392.x (2006).
Munakata, M. et al. Normal sympathetic vasomotor and cardiac parasympathetic activities in patients with primary aldosteronism: assessment by spectral analysis. Journal of the autonomic nervous system 52, 213–223 (1995).
Maser, R. E., Lenhard, M. J., Kolm, P. & Edwards, D. G. Direct renin inhibition improves parasympathetic function in diabetes. Diabetes, obesity & metabolism 15, 28–34 (2013).
Munakata, M. et al. Increased gain in baroreceptor-heart rate reflex in patients with primary aldosteronism. J Hypertens 13, 1648–1653 (1995).
Catena, C. et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 50, 911–918 (2007).
Strauch, B. et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens 21, 1086–1092 (2008).
Abad-Cardiel, M. et al. Hypertension caused by primary hyperaldosteronism: increased heart damage and cardiovascular risk. Revista espanola de cardiologia 66, 47–52 (2013).
Kuo, C. C. et al. Verification and evaluation of aldosteronism demographics in the Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group). J Renin Angiotensin Aldosterone Syst 12, 348–357 (2011).
Hung, C. S. et al. Twenty-four-hour urinary aldosterone predicts inappropriate left ventricular mass index in patients with primary aldosteronism. The Scientific World Journal 2013, 294594 (2013).
Lee, H. H. et al. Myocardial ultrasound tissue characterization of patients with primary aldosteronism. Ultrasound in medicine & biology 39, 54–61 (2013).
Vanoli, E. et al. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation 91, 1918–1922 (1995).
Peng, C. K., Havlin, S., Stanley, H. E. & Goldberger, A. L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87 (1995).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. American journal of physiology. Heart and circulatory physiology 278, H2039–2049 (2000).
Wu, Z., Huang, N. E., Long, S. R. & Peng, C. K. On the trend, detrending and variability of nonlinear and nonstationary time series. Proceedings of the National Academy of Sciences of the United States of America 104, 14889–14894 (2007).
Peng, C. K., Costa, M. & Goldberger, A. L. Adaptive Data Analysis of Complex Fluctuations in Physiologic Time Series. Advances in adaptive data analysis 1, 61–70 (2009).
Lo, M. T., Novak, V., Peng, C. K., Liu, Y. & Hu, K. Nonlinear phase interaction between nonstationary signals: a comparison study of methods based on Hilbert-Huang and Fourier transforms. Physical review. E, Statistical, nonlinear and soft matter physics 79, 061924 (2009).
Wu, V. C. et al. Kidney impairment in primary aldosteronism. Clin Chim Acta 412, 1319–1325 (2011).
Yen, R. F. et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med 50, 1631–1637 (2009).
Wu, V. C. et al. Diagnosis and Management of Primary Aldosteronism. Acta Nephrologica 26, 111–120 (2012).
Devereux, R. B. & Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55, 613–618 (1977).
Acknowledgements
We thank Ms. Fen-Fun Hsien for medical technologic support and the staff of the Second Core Lab of Department of Medical Research in National Taiwan University Hospital for technical assistance. This study was supported by National Taiwan University Hospital (grants NTUH 102-S2096, UN102-060), National Taiwan University (National Taiwan University Cutting-Edge Steering Research 10R71608-1), Ministry of Science and Technology (NSC 100-2314-B-002-139, NSC 102-2314-B-002-078-MY3, MOST 103-2220-E-002-011, MOST 103-2218-E-008-006, MOST 103-2221-E-008-006-MY3), Ministry of Science and Technology support for the Center for Dynamical Biomarkers and Translational Medicine, National Central University, Taiwan (NSC 102-2911- I-008-001) and the joint foundations (Grant No 103CGH-NCU-A1, VGHUST103-G1-3-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.H.L., K.D.W., C.K.P. and Y.L.H. conceived and designed the experiments. V.C.W, M.T. L., X.M.W. and C.S.H. collected and analyzed the data. Y.H.L. and C.L. wrote the paper. C.L., M.S. and C.K.P. made scientific comment on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, YH., Wu, VC., Lo, MT. et al. Reversible heart rhythm complexity impairment in patients with primary aldosteronism. Sci Rep 5, 11249 (2015). https://doi.org/10.1038/srep11249
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11249
This article is cited by
-
Heart rhythm complexity analysis in patients with inferior ST-elevation myocardial infarction
Scientific Reports (2023)
-
Real-time machine learning model to predict in-hospital cardiac arrest using heart rate variability in ICU
npj Digital Medicine (2023)
-
Heart rhythm complexity as predictors for the prognosis of end-stage renal disease patients undergoing hemodialysis
BMC Nephrology (2020)
-
Usefulness of heart rhythm complexity in heart failure detection and diagnosis
Scientific Reports (2020)
-
Alterations in heart-brain interactions under mild stress during a cognitive task are reflected in entropy of heart rate dynamics
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.