Abstract
Regulatory T (Treg) cells are characterized by the expression of CD4, CD25 and the intracellular Foxp3. However, these markers do not indicate whether Treg cells are thymic derived Treg (tTreg) cells or peripherally induced Treg (pTreg) cells. Recently, Helios and Neuropilin-1 (Nrp1) has been reported as potential markers for tTreg cells. Herein, we used flow cytometry to examine the proportion of CD4+CD8−CD25+ Treg cells expressing Helios, Nrp1 and Foxp3 in thymus, pancreatic draining lymph nodes (PDLNs) and spleen of CD-1 mice and thymus of NOD and C57BL/6 mice. The frequency of Helios+ cells was higher than that of Nrp1+ cells in CD4+CD8−CD25+ and CD4+CD8−CD25+Foxp3+ Treg cells in thymus. Interestingly, the proportion of IL-10+, Ebi3+and CTLA-4+ cells was higher in Helios+ than Nrp1+ tTreg cells. The anti-apoptotic activity of Helios+ tTreg cells was higher in thymus compared to Nrp1+ tTreg cells. Nrp1 seems to be expressed at a later developmental stage compared to Helios and Foxp3. Furthermore, the expression of Nrp1 in CD4+CD25+ T cells of younger mice did not increase after stimulating them in vitro with anti-CD3 and –CD28. Thus, under these conditions, Helios could be considered a more reliable marker for distinguishing tTreg cells from pTreg cells than Nrp1.
Similar content being viewed by others
Introduction
Regulatory T (Treg) cells play a pivotal role in maintaining the homeostasis of the immune system by; (1) secreting anti-inflammatory cytokines such as: interleukin-10 (IL-10), IL-35 and transforming growth factor-β (TGF-β), (2) producing granzyme A or B (3) increasing in the consumption of IL-2 to destruct effector T cells by metabolic disruption and (4) enhancing the dendritic cells to produce indoleamine 2,3-dioxygenase to suppress the effector T cells (reviewed in ref. 1–3)1,2,3. Treg cells express CD4 and CD25 in naïve conditions4,5 and despite intensive research in the field of Treg cells, Foxp3 is still (together with CD4 and CD25) the main marker for detection of these cells6,7,8. There are several other markers that are also expressed by Treg cells such as CD103, CTLA-4, ICOS, glucocorticoid induced TNF-related protein (GITR), programmed cell death protein 1 (PD-1) and Swap704,9,10,11. However, these markers are unable to distinguish between thymic derived or natural Treg (tTreg) cells and peripherally induced Treg (pTreg) cells. Also, some of these markers (eg. CD103, CTLA-4, ICOS and PD-1) are upregulated in activated CD4+ T cells4,9,12,13.
In 2010, Thornton et al. have reported that Helios, a member of the Ikaros family, is expressed by tTreg cells and that Helios could be used as a marker for distinguishing between tTreg cells and pTreg cells14. Recently, two other groups reported that Neuropilin-1 (Nrp1), a semaphorin III receptor, could be used as a marker for tTreg cells under certain conditions11,15. Nrp1 was also earlier reported as a cell surface marker for mouse, but not human, Treg cells16,17.
In the present study, we have extended a serendipitous observation of ours; we found that not all the Foxp3+ Treg cells in thymic glands of naïve mice were expressing Nrp1, but all were expressing Helios. To further substantiate, we examined CD4+CD8−CD25+ Treg cells and used flow cytometry to compare the expression of the three different markers Foxp3, Helios and Nrp1 on CD4+CD8−CD25+ Treg cells derived from thymus, pancreatic draining lymph nodes (PDLNs) and spleen. We found that both Helios and Nrp1 are markers for tTreg cells as earlier reported11,15, but Helios is expressed in a higher proportion of tTreg cells than Nrp1. In addition, we found that there is a higher proportion of Epstein-barr virus induced gene 3+ (Ebi3) (a subunit of IL-35 cytokine), IL-10+ and cytotoxic T-lymphocyte associated protein 4+ (CTLA-4) cells among Helios+ tTreg cells than among Nrp1+ tTreg cells, indicating that Helios+ tTreg cells are more functionally active. Also, the anti-apoptotic activity of Helios+ tTreg cells was higher than that of Nrp1+ tTreg cells. According to our findings, it seems that Helios might, under certain conditions, be more suitable than Nrp1 to use as a marker for distinguishing tTreg cells.
Results
Nrp1 distinguishes between tTreg cells and pTreg cells to some extent
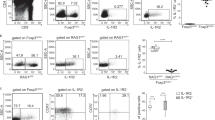
It has been reported that Nrp1 is a marker for Treg cells and also helps in distinguishing between tTreg cells and pTreg cells in mice11,15,16. To further elucidate this issue the frequency of CD4+CD8−CD25+ Treg cells expressing Nrp1 and Foxp3 were analysed in CD-1 mice. These mice were used in this study as this mouse strain is widely used as an outbred wild type animal strain18. We found that 30%, 49% and 49% of CD4+CD8−CD25+ cells were Foxp3+Nrp1+ in thymus, PDLNs and spleen, respectively (Fig. 1A). Interestingly, similar proportions of CD4+CD8−CD25+Foxp3+ Nrp1− (Foxp3+Nrp1−) Treg cells were found in the thymus, PDLNs and spleen (Fig. 1A). However, very few of the CD4+CD8−CD25+ cells were Foxp3−Nrp1+ in PDLNs and spleen, while 17% of the CD4+CD8−CD25+ cells were Foxp3−Nrp1+ in thymus (Fig. 1A). These results indicate that not all the CD4+CD8−CD25+Foxp3+ (Foxp3+) Treg cells express Nrp1. Accordingly, we suggest that CD4+CD8−CD25+Foxp3+Nrp1+ (Foxp3+Nrp1+) Treg cells are either tTreg cells or that the expression of Nrp1 indicate a further differentiation state of Treg cell development.
Nrp1 distinguishes between tTreg cells and pTreg cells.
CD-1 Mouse thymus, PDLNs and spleen cells were stained for CD4, CD25, CD8, Nrp1, Foxp3 and Helios (gating strategies are shown in Fig. 6A–F). CD4+CD8−CD25+ cells were gated based on CD4, CD8 and CD25 expression. CD4+CD8−CD25+ cells were gated and further analysed for Nrp1 and Foxp3 expression by flow cytometry (A). CD4+CD8−CD25+Foxp3+Nrp1+ (black bar), CD4+CD8−CD25+Foxp3+Nrp1− (grey bar) and CD4+CD8−CD25+Foxp3−Nrp1+ (white bar) T cells were analysed for Helios expression using flow cytometry (gating strategies are shown in Fig. 3 A–C) (B). Results are expressed as means ± SEM, from two experiments (n = 4–5 mice/experiment). Unpaired t-tests were performed for comparisons between the groups. * and *** denote p < 0.05 and p < 0.001, respectively.
Subsequently, we analysed the frequency of Helios+ and CD44+ cells in the three different cell populations (Foxp3+Nrp1+, Foxp3+Nrp1− and Foxp3−Nrp1+ cells). We monitored CD44 to investigate the maturity of T cells as this marker is widely used for differentiating the T cells according to their maturity stage, namely, memory T cells19. The proportions of CD44+ cells in all the studied tissues were similar in both Foxp3+Nrp1+ Treg cells and Foxp3+Nrp1− Treg cells (~90%; data not shown). We found that 97%, 78% and 85% of Foxp3+Nrp1+ Treg cells were expressing Helios in thymus, PDLNs and spleen, respectively (Fig. 1B). The proportions of Helios+ cells were higher in Foxp3+Nrp1+ than in Foxp3+Nrp1− Treg cells in thymus, PDLNs and spleen (Fig. 1B). These results illustrate that Nrp1 can be used to distinguish between tTreg and pTreg cells as previously reported11,15. Thus, our data confirm and extend the previous findings that Nrp1 can be used as a marker for tTreg cells but maybe is less useful as a marker for pTreg cells.
Nrp1 may not be a suitable marker under certain conditions for distinguishing tTreg cells
Our results support the hypothesis that Foxp3+Nrp1+ cells are tTreg cells. However, we noticed that 80%, 41% and 52% of the Foxp3+Nrp1− Treg cells in thymus, PDLNs and spleen, respectively, were expressing Helios (Fig. 1B). Earlier, Foxp3+Nrp1− cells have been described as pTreg cells11,15, which raised the question of whether Nrp1 is a good marker for distinguishing between tTreg and pTreg cells. On the other hand, as many as 74%, 48% and 100% Foxp3−Nrp1+ T cells were Helios+, which is more than would be expected if both Nrp1 and Helios are markers for distinguishing tTreg cells (Fig. 1B). Since Foxp3−Nrp1+ cells are described as activated T cells and Thornton et al. has shown that up to 5–8% T cells can express Helios11,14,15, we therefore, hypothesize that either Nrp1 or Helios is not an optimal marker to detect tTreg cells.
To further explore this we analysed the frequencies of Nrp1+, Helios+ or Foxp3+ cells separately in CD4+CD8−CD25+ Treg cells. The proportion of Helios+ cells (77%) was higher than the proportion of Nrp1+ (38%) and Foxp3+ (52%) cells in thymus (Fig. 2A). The proportion of Helios+ cells in CD4+CD8−CD25+ Treg cells was higher in thymus compared to PDLNs and spleen (Fig. 2A, thymus vs PDLNs p < 0.001, not indicated in the figure). The proportion of Nrp1+ cells in CD4+CD8−CD25+ Treg cells did not differ much between the three organs, but in spleen these cells were slightly fewer than in thymus (Fig. 2A, p < 0.05, not indicated in the figure). The Foxp3+ cell proportion in CD4+CD8−CD25+ Treg cells was higher in PDLNs than in thymus and spleen (Fig. 2A, thymus vs PDLNs p < 0.001 and PDLNs vs spleen p < 0.05, not indicated in the figure). The higher proportion of Foxp3+ Treg cells in PDLNs could be due to the fact that PDLNs control the immune system of the pancreas and represent a local immune system, whilst the thymus is a central immune organ and spleen reflects a systemic immune response. Furthermore, this could also be the explanation for having 70% Helios+ cells in CD4+CD8−CD25+ Treg in spleen (Fig. 2A).
Nrp1 may not be a suitable marker under certain conditions for distinguishing tTreg cells.
The upper panel shows an example (CD-1 thymocytes) of the strategies for cells analysed in A and B (left panels) and C and D (right panels). CD4+CD8−CD25+ cells of CD-1 mice were further analysed for individual expression of Nrp1, Helios or Foxp3 in thymus, PDLNs and spleen cells using flow cytometry (A). Thymic glands of NOD and C57BL/6 mice were examined similarly (C). Subsequently, CD4+CD8−CD25+ cells were gated first for Foxp3 and then CD4+CD8−CD25+Foxp3+ cells were analysed for either Helios or Nrp1 expression using FACS in CD-1 mice (B) and in thymus of NOD and C57BL/6 mice (D). Results are expressed as means ± SEM, from two experiments (n = 4–5 mice/experiment). *, ** and *** denote p < 0.05, p < 0.01 and p < 0.001, respectively. One-way ANOVA (A, B and C) and unpaired t-tests (D) were performed for comparisons.
Lio et al. have shown that ~20% of CD4+CD8−CD25high thymocytes develop into Foxp3+ Treg cells when injected intrathymically into mouse recipients20. Furthermore, Lio et al. suggested that not all the CD4+CD8−CD25high cells develop into Foxp3+ Treg cells in the thymus. Therefore, we analysed the proportions of Helios+ or Nrp1+ cells in Foxp3+ Treg cells in thymus, PDLNs and spleen. In thymus the proportion of Helios+ cells (95%) was significantly higher than that of Nrp1+ cells (47%) (Fig. 2B). The same pattern was also seen in PDLNs and spleen (Fig. 2B). The proportion of Helios+ cells among Foxp3+ Treg cells was highest in thymus, followed by spleen and lowest in PDLNs (Fig. 2B). The Helios+ cell proportion observed in Foxp3+ Treg cells in thymus, PDLNs and spleen were very similar to previous findings14. These results indicate that Helios might be a better marker for detection of tTreg cells compared to Nrp1.
This notion was further supported when we examined the proportions of Foxp3+, Nrp1+ or Helios+ cells in CD4+CD8−CD25+ Treg cells in the thymic glands of NOD and C57BL/6 mice and got similar results in both strains (Fig. 2C). In addition, the proportion of Helios+ cells (100%) was higher than that of Nrp1+ cells (~60%) in Foxp3+ in the thymus of NOD and C57BL/6 mice (Fig. 2D). In this study, we used NOD and C57BL/6 mice to further show that Helios expression is not mouse strain specific. The NOD murine model is widely used as spontaneous experimental mouse model for type 1 diabetes and was used to represent an autoimmune state, while C57BL/6 was used as a wild type mouse strain.
In thymus, 0.5% of CD4+CD8−CD25− T cells can develop into Foxp3+ Treg cells20. Thus, we analysed Helios and Nrp1 expression separately in CD4+CD8−CD25−Foxp3+ Treg cells to investigate the expression of these markers in the thymus. Interestingly, Helios expression (~100%) was much higher than Nrp1 expression (between ~0 and 30%) in the thymic glands of CD-1, NOD and C57BL/6 mice (Fig. 3).
Helios could be used as a tTreg cell marker in both CD-1 and NOD mice.
The upper panel shows an example of the gating strategies for CD-1 lymphocytes in lower panel. Thymic CD4+CD8−CD25−Foxp3+ cells of CD-1 mice were further analysed for individual expression of Nrp1 and Helios in thymus and thymic glands of NOD and C57BL/6 mice were examined similarly (lower panel). Results are expressed as means ± SEM, from two experiments (n = 4–5 mice/experiment). *** denotes p < 0.001, unpaired t-tests were performed for comparisons.
Since Nrp1 is a transmembrane protein21,22, we investigated the intracellular staining of Nrp1 and found that approximately 50–60% of CD4+CD25+Foxp3+ Treg cells were expressing Nrp1 intracellularly. These results also indicate that, Helios may be a more reliable marker than Nrp1 for detection of the tTreg cells in naïve conditions.
Helios+ tTreg cells have a higher frequency of IL-10, Ebi3 and CTLA-4 than Nrp1+ tTreg cells
We found that Helios seems to be a better marker for tTreg under certain conditions (Fig. 2 and 3). A question in this context is if this is reflected in the functional capacity of the Helios+ and Nrp1+ tTreg cells. We therefore determined the proportions of IL-10+, Ebi3+ (a subunit of IL-35 cytokine) and CTLA-4+ cells among Foxp3+Helios+ and Foxp3+Nrp1+ tTreg cells. Treg cells secrete anti-inflammatory cytokines (IL-10, IL-35 and TGF-β) to suppress ongoing immune assaults (reviewed in ref. 1–3)1,2,3. Furthermore, several reports support the hypothesis that CTLA-4 expressing Treg cells are highly suppressive (reviewed in ref. 2)2. Interestingly, we found that the proportions of IL-10+ and Ebi3+ cells were higher in Helios+ tTreg cells than in Nrp1+ tTreg cells of thymic glands, PDLNs and spleen (Fig. 4A–B and Supplementary Fig. 1–2). Also, the proportion of CTLA-4+ cells was higher in Helios+ tTreg compared to Nrp1+ tTreg cells of thymic glands and spleen, but not in PDLNs (Fig. 4C and Supplementary Fig. 3). However, the expression of CTLA-4, as indicated by florescence intensity, was higher in Helios+ tTreg than Nrp1+ in PDLNs and spleen, but not in thymic glands (Fig. 4D). Thus, our results illustrate that the functional activity of Helios+ tTreg cells is higher than that of Nrp1+ tTreg cells.
Helios+ tTreg cells are more functionally active than Nrp1+ tTreg cells.
The proportion of (A) IL-10+, (B) Ebi3+ and (C) CTLA-4+ cells in CD4+CD8−CD25+Foxp3+Nrp1+ (grey bars) or CD4+CD8−CD25+Foxp3+Helios+ (black bars) cells of thymic glands, PDLNs and spleen were analysed by using flow cytometry (gating strategies are shown in Suppl. fig. 1-3). (D) Mean fluorescence intensity (MFI) of CTLA-4 in CD4+CD8−CD25+Foxp3+Nrp1+ (grey bars) or CD4+CD8−CD25+Foxp3+Helios+ (black bars) cells of thymic glands, PDLNs and spleen were analysed by using flow cytometry. (E) Representative histograms showing the expression of CTLA-4 in CD4+CD8−CD25+Foxp3+Nrp1+ (grey shaded area) or CD4+CD8−CD25+Foxp3+Helios+ (open area)39 cells of thymus, PDLNs and spleen. Results are expressed as means ± SEM, from two experiments (n = 2 mice/experiment). Unpaired t-tests were performed for comparisons between the groups. *, ** and *** denote p < 0.05, p < 0.01 and p < 0.001, respectively.
The anti-apoptotic activity of Nrp1+ tTreg cells is lower than that of Helios+ tTreg cells in thymic glands
We found that Helios probably is a better marker than Nrp-1 for distinguishing tTreg cells from pTreg cells and that there are more IL-10+, Ebi3+ and CTLA-4+ cells among Helios+ tTreg cells. Delgoffe et al. have reported that Nrp1 is required for the stability and functionality of Treg cells23. However, the expression of Bcl-2 is often used to determine the apoptotic stability or anti-apoptotic activity of Treg cells24 and we therefore analysed the expression of Bcl-2 in Helios+ and Nrp1+ tTreg cells using flow cytometry. We found that the expression of Bcl-2 was higher in Helios+ tTreg in Nrp1+ tTreg cells of thymic glands (Fig. 5A–B). However, the expression of Bcl-2 was equal in Helios+ and Nrp1+ tTreg cells of PDLNs and spleen (Fig. 5A–B). Thus, our data demonstrate that the anti-apoptotic activity of Helios+ tTreg cells in thymic glands is higher than in Nrp1+ tTreg cells.
Helios+ tTreg cells are more anti-apoptotic active than Nrp1+ tTreg cells in thymus.
(A) Representative histograms showing the expression of Bcl-2 in CD4+CD8−CD25+Foxp3+Nrp1+ (grey shaded area) or CD4+CD8−CD25+Foxp3+Helios+ (open area)39 cells of thymus, PDLNs and spleen (gating strategies are shown in Suppl. fig. 1–3). (B) Mean fluorescence intensity (MFI) of Bcl-2 in CD4+CD8−CD25+Foxp3+Nrp1+ (grey bars) or CD4+CD8−CD25+Foxp3+Helios+ (black bars) cells of thymic glands, PDLNs and spleen were analysed by flow cytometry. Results are expressed as means ± SEM, from one experiment (n = 4). Unpaired t-tests were performed for comparisons between the groups, ** denotes p < 0.01.
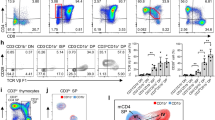
Nrp1 is not expressed by all tTreg cells
Our results suggest that Nrp1 may not be an ideal marker for detection of tTreg cells (Fig. 2 and 3), since all tTreg cells might not express Nrp1. To further investigate this we analysed the proportion of CD4+CD8−CD25+Foxp3+Helios− (Foxp3+Helios−) Treg cells and Foxp3+Nrp1− Treg cells in CD4+CD8−CD25+ T cells. These two different populations are considered to represent pTreg cells11,14,15. In CD-1 mice 22%, 43% and 38% of CD4+CD8−CD25+ Treg cells were Foxp3+Nrp1− in thymus, PDLNs and spleen, respectively (Fig. 6A–C and G). On the other hand, Foxp3+Helios− cells constituted 6% in thymus, 36% in PDLNs and 26% in spleen of the CD4+CD8−CD25+ Treg cells (Fig. 6B-F and G).
Nrp1 is not expressed by all tTreg cells.
The upper panels (A–F) shows an examples of the gating strategies for CD-1 mouse thymocytes, PDLN cells and splenocytes analysed in lower panels (G–L). CD4+CD8−CD25+ cells were gated for Foxp3+Helios− or Foxp3+Nrp1− cells (G), Foxp3+Nrp1+ or Foxp3+Helios+ cells (I) and Foxp3−Nrp1+ or Foxp3−Helios+ cells (K) by flow cytometry in CD4+CD8−CD25+ Treg cells of thymus, PDLNs and spleen. CD4+CD8−CD25+ cells from thymus, PDLN and spleen were gated first for Foxp3 and Helios and then the proportions of Nrp1+ cells in the different populations (CD4+CD8−CD25+Foxp3+Helios− (H), CD4+CD8−CD25+Foxp3+Helios+ (J) and CD4+CD8−CD25+Foxp3−Helios+ (L) cells) were analysed. Alternatively, CD4+CD8−CD25+ cells were first gated for Foxp3 and Nrp1 and then the proportion of Helios+ cells were measured in CD4+CD8−CD25+Nrp1−Foxp3+ (H), CD4+CD8−CD25+Nrp1+Foxp3+ (J) and CD4+CD8−CD25+Nrp1+Foxp3− (L) cells. Results are expressed as means ± SEM, data shown from two experiments (n = 4–5 mice/experiment) and *, ** and *** denote p < 0.05, p < 0.01 and p < 0.001, respectively. Unpaired t-tests were used for comparisons.
Next, we analysed the proportions of Nrp1+ cells in Foxp3+Helios− pTreg cells and Helios+ cells in Foxp3+Nrp1− pTreg cells (Fig. 6A–F and H). Interestingly, there were 0%, 14% and 5% Nrp1+ cells in Foxp3+Helios− pTreg cells in thymus, PDLNs and spleen, respectively (Fig.6 H). On the contrary, 80%, 41% and 52% of Foxp3+Nrp1− pTreg cells were Helios+ (Fig. 6H). These results indicate that when Helios is used as a tTreg cell marker a higher percentage of tTreg cells are detected than when using Nrp1 as a marker.
CD4+CD8−CD25+Foxp3+Helios+ (Foxp3+Helios+) and CD4+CD8−CD25+Foxp3+Nrp1+ (Foxp3+Nrp1+) T cells are characterized as tTreg cells11,14,15. The proportion of Foxp3+Helios+ Treg cells was higher than the proportion of Foxp3+Nrp1+ among CD4+CD8−CD25+ Treg cells in all three investigated tissues (Fig. 6A–F and I). Nrp1+ cells constituted a smaller percentage of Foxp3+Helios+ than Helios+ cells of Foxp3+Nrp1+ cells (Fig. 6A–F and J). In line with this, Weiss et al. has shown that Helios mRNA is expressed in higher amounts in a Nrp1high Treg cell population than Nrp1 mRNA11. Nrp1+ cells were fewer in CD4+CD8−CD25+Foxp3−Helios+ cells compared to Helios+ cells in CD4+CD8−CD25+Foxp3−Nrp1+ cells (Fig. 6A–F and K–L). One can speculate that Foxp3+Nrp1− or Helios+Nrp1− Treg cells are immature cells since Weiss et. al, have reported that when thymic Foxp3lowNrp1− and Foxp3+Nrp1+ cells were injected to Thy1 congenic WT mice, 60% of the Foxp3lowNrp1− cells upregulated their Nrp1 expression11. Recently, Mahmud et al. have reported that the tumor-necrosis factor receptor (TNFR) superfamily members GITR, OX40 and TNFR2 are required for the differentiation of tTreg cells25. Therefore, we analysed the expression of GITR on both Helios+ and Nrp1+ tTreg cells of adult mice. The expression of GITR on Helios+ was equal to that on Nrp1+ Treg cells (Supplementary fig. 4A), suggesting that the Helios+ and Nrp1+ tTreg cell populations were equally mature. These combined data show that almost all the Nrp1+ cells express Helios, but all the Helios+ cells do not express Nrp1.
To further advocate that all the Foxp3+ tTreg cells do not express Nrp1 we determined the proportions of Nrp1−Helios+, Nrp1+Helios+, Nrp1−Helios− and Nrp1−Helios+ cells amongst thymic Foxp3+ tTreg cells. We found that among Foxp3+ tTreg cells 1 ± 0.1%, 47 ± 4%, 2 ± 0.78% and 49 ± 4% of Foxp3+ tTreg cells were Nrp1−Helios+, Nrp1+Helios+, Nrp1−Helios− and Nrp1−Helios+, respectively. These results indicate that Nrp1 is not expressed by all the thymic Foxp3+ tTreg cells.
Nrp1 expression starts at a later stage of the T cells development
We confirmed that all the Helios+ tTreg cells were not expressing Nrp1 (Fig. 6) and that the apoptotic stability of Helios+ tTreg cells was higher than Nrp1+ tTreg cells in thymic glands (Fig. 5). This could be because the Nrp1 expression starts later in the development of T cells compared to Helios and Foxp3 expression. This notion is supported by our study of Foxp3, Nrp1 and Helios expression in CD4+CD8− T cells in thymus and spleen of 7 days old NOD mice. We did not see any CD4+CD8− T cells expressing Nrp1, but at the same time these cells were expressing Foxp3 and Helios (Fig. 7A–F). In addition, CD4+CD8−CD25+Foxp3+Helios+ Treg cells of 7 days old mice were expressing GITR (Supplementary fig. 4B), indicating that these cells are mature cells and that the lack of Nrp-1 expression is not a sign of an immature status of the cells. We also investigated the expression in 3, 5, 7, 10 and 14 days old NOD mice and could confirm that the expression of Nrp1 in thymic CD4+CD8−CD25+Foxp3+ Treg cells first starts between day 7 and 10 after birth (Figure 7A–F and Supplementary fig. 5).
Nrp1 expression starts at a later stage of the T cell development.
Thymocytes and splenocytes from seven days old NOD mice were analysed for the expression of Foxp3, Helios and Nrp1 during T cell development (A–F) using flow cytometry. CD4+CD8− T cells were gated based on CD4 and CD8 expression, then further analysed for the expression of Foxp3, Helios and Nrp1. CD4+CD25− and CD4+CD25+ T cells were sorted from thymic glands of 7 days old mice, stimulated with anti-CD3 and anti-CD28 as described in Methods section. G–L shows the expression of Nrp1 or Helios in the indicated cell populations. Dot plots are representative of two experiments (n = 2–4 mice/experiment).
Bruder et al. have shown that the expression of Nrp1 was enhanced on CD4+ T cells by stimulating the CD4+ T cells in vitro by anti-CD3 and anti-CD28, suggesting that T cell receptor (TCR) signalling can induce the expression of Nrp116. Also, recently Levin et al. have reported that the TCR play a pivotal role in the suppressive function of Treg cells26. To investigate the possibility of an effect of Nrp1 on the TCR expression, we stimulated CD4+CD25− T and CD4+CD25+ Treg cells isolated from thymic glands of 7 days old NOD mice as described in Methods section. We did not find any upregulation of Nrp1 expression in either CD4+CD25− T and CD4+CD25+ Treg cells (Fig. 7G–J) after TCR stimulation. The expression of Helios was not altered after the stimulation (Fig. 7K–L). In this study, we used 7 days old NOD mice since Helios has shown a similar response in the thymus of NOD mice as in CD-1 mice thymus.
Discussion
The Ikaros family member Helios and semaphorin III receptor Nrp1 have been suggested as potential markers for distinguishing tTreg cells from pTreg cells11,14,15. However, in the present study, we found that the proportion of Helios+ cells was higher in thymic CD4+CD8−CD25+ and Foxp3+ Treg cells than that of Nrp1+ cells. These data illustrate that Helios may be a more suitable marker for distinguishing tTreg cells than Nrp1. In addition, we found that Helios+ treg cells were having a higher proportion of IL-10+, Ebi3+ and CTLA-4+ cells than those of Nrp1+ tTreg cells. In this study we used the CD-1 mice, which is an outbred experimental model18. Furthermore, to affirm that our data from the CD-1 mice was not strain biased, we investigated NOD and C57BL/6 mice. Interestingly, all the different mouse strains studied in this context showed a similar response. To the best of our knowledge this is the first study in vivo where Foxp3, Helios and Nrp1 have been investigated simultaneously in the thymic glands of naïve mice. However, Schliesser et al., have investigated in vitro co-expression of Nrp1 or Helios in Foxp3+ alloreactive Treg (aTreg) cells generated from CD4+ T cells co-cultured with TGF-β, anti–CD4 antibody and retinoic acid or rapamycin in vitro27. Their data are in line with our finding since they also found that 70% of Foxp3+ aTreg cells were expressing Helios and only 53% of Foxp3+ aTreg cells were co-expressing Nrp1. This could be expected since the CD4+ T cells were isolated from spleen and LNs to generate aTreg cells and it has been reported that almost 70% of Foxp3+ Treg cells are Helios+ in peripheral organs14.
In the present study, we found that Foxp3+Nrp1+ Treg cells in the peripheral tissues PDLNs and spleen were Helios+. These results further confirmed that Nrp1 could be used as a marker for tTreg cells11,15. However, as much as 80%, 41% and 52% of the Foxp3+Nrp1− pTreg cells were expressing Helios in thymus, PDLNs and spleen, respectively. One can speculate that our findings could be due to recirculation of pTreg cells to the thymus from the periphery. Helios expression has been reported in pTreg cells earlier28. If that is the case, then 4–7% of CD4+Foxp3− cells can be converted into CD4+Foxp3+ in peripheral tissue29. In our study, Foxp3+Helios− pTreg cells constituted only 6% of the cells in the thymic glands of CD-1 mice. On the other hand Foxp3+Nrp1− pTreg cells constituted as many as 22% of the thymic cells. These results illustrate that when Nrp1 is used as a tTreg cell marker, a higher proportion of pTreg cells are detected in the thymus. This is in contrast to what one would expect in the thymus since it has been reported that the Treg cell population in the thymus is constituted of between 0 to 10% pTreg cells and in periphery ~30% of Treg cells are constituted of pTreg cells14. However, the 6% of Foxp3+Helios− pTreg cells in thymic glands of CD-1 mice is in good agreement with previous determinations of CD4+CD8−Foxp3+Helios− (8–10%) in the thymic glands of 8 weeks old normal mice14. It is conceivable that the presence of Foxp3+Helios− cells represent pTreg cells reaching the thymus via recirculation. Up to 5% of activated T cells can recirculate to the thymus from the periphery30. The 6% of Foxp3+Helios− pTreg cells we detected in CD-1 mice thymic glands corresponds well with these data. Furthermore, Ayyoud et al. have suggested that <10% Foxp3+Helios− Treg cells can be found in the human cord blood31. In line with this finding, MacDonald et al. have reported that ~17% of Foxp3+Helios− Treg cells exist in the human thymus. However, when Foxp3+Helios− Treg cells in human thymus were further gated for a naïve T cell marker, the proportion of Foxp3+Helios− Treg cells was reduced to ~3%32. Our findings and later studies reveal that 22% of Foxp3+Nrp1− pTreg cell is an unusually high number.
Approximately 80% of the thymic Foxp3+Nrp1− pTreg cells were expressing Helios, but the Foxp3+Helios− pTreg cells were not expressing Nrp1. This indicates that when Nrp1 is used as a marker for tTreg cell, Nrp1 does not detect many of the tTreg cells, but when Helios is used as a marker for tTreg cells, most of the tTreg cells are detected. Moreover, we did not detect any Foxp3+Helios− pTreg cells in the thymic glands of NOD and C57BL/6 and the proportions of Foxp3+Helios− Treg cells in thymus, PDLNs and spleen of CD-1 mice are in line with previous results14.
Weiss et. al, has reported that when thymic Foxp3lowNrp1− and Foxp3+Nrp1+ cells were injected to Thy1 congenic WT mice, 60% of the Foxp3lowNrp1− cells upregulated their Nrp1 expression11. These data further support our finding that the in vivo environment can upregulate the Nrp1+ expression of pTreg cells. One can also speculate that Foxp3+Nrp1− or Helios+Nrp1− Treg cells are immature cells, but in the present study we found that the expression of GITR on Helios+ Treg cells was equal to that on Nrp1+ Treg cells, indicating that Helios+Nrp1− Treg cells are not immature cells. In addition, CD44 expression was also equal in Foxp3+Nrp1+ and Foxp3+Nrp1− Treg cells, suggesting that both of these Treg cells were mature. Taken together, our results support our hypothesis that Helios is a more suitable marker for tTreg cells than Nrp1. Also, Nrp1 has been suggested not to be a good marker to detect human Treg cells17.
The expression of Helios has been reported to increase in activated and strongly autoreactive T cells, a finding that further challenge the suitability of Helios as a tTreg cell marker33,34. Nevertheless, it has also been reported that Nrp1 is required for stability and functionality of Treg cells23. On the other hand, we found in the present study that the apoptotic stability of Helios+ tTreg cells was higher than those of Nrp1+ in thymic glands. Furthermore, there were a higher proportion of IL-10+, Ebi3+ (a subunit of IL-35 cytokine) and CTLA-4+ cells among Helios+ tTreg cells than among Nrp1+ tTreg cells. These data indicate that the functional activity of Helios+ tTreg cells is higher than Nrp1+ tTreg cells, a notion that was further supported by Getnet et al., who showed that the knockdown of Helios by siRNA in human CD4+CD25+ Treg cells decreases the suppressive activity of Treg cells35.
Altogether, our results indicate that both Helios and Nrp1 can be used as markers for tTreg cells, but that Nrp1 seems less suitable as a marker compared to Helios in naïve conditions. It could be that Nrp1 expression starts at a later stage of the T cell development since it has been reported that Helios expression precedes the Foxp3 expression in thymus14, a notion that is supported by our findings that Nrp1 is expressed after the Helios and Foxp3 in the Treg cells. An equal expression of GITR on Treg cells of 7 days old mice compared to Treg cells of adult mice suggest that the Treg cells in 7 days old mice were mature.
The delayed expression of Nrp1 on Treg cells led to the question of whether the expression of Nrp1 could be regulated by the TCR. In the present study, we found that TCR stimulation did not upregulate the expression of Nrp1, disproving this idea.
Furthermore, the anti-apoptotic activity of Nrp1+ tTreg cells is lower compared to Helios+ tTreg cells in the thymic glands and it seems that Helios+ tTreg cells are functionally more active than Nrp1+ tTreg cells. On the other hand, Nrp1 is an angiogenesis factor and it is involved in enhancing a strong interaction between Treg cells and dendritic cells36,37, which could be another reason that Nrp1 is not expressed by all the tTreg cells.
Methods
Animals
Male CD-1 mice were obtained from Charles River (Hannover, Germany). Female C57BL/6 mice were obtained from Taconic (Bomholt, Denmark). The NOD mice used were originally obtained from the Clea Company (Aobadi, Japan) and have subsequently been inbred under pathogen-free conditions at the animal department, Biomedical Center, Uppsala, Sweden. The use of animals was in accordance with international guidelines (NIH publications 85–23) and the local animal ethics committee at Uppsala University approved the animal experiments.
Cell isolation from thymic glands, PDLNs and spleens
Single cell suspensions of spleen tissue were made as previously described38. Also, single cell suspensions of thymic glands were prepared in the same manner as spleen cell suspensions. PDLNs were dissected and ground through a mesh to release the cells and then washed with RPMI medium (Sigma Aldrich, St Louis, MO, USA). The cell suspensions were washed, all the cell suspensions were counted using FACSCalibur (Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA) and 1 × 106 cells were used for flow cytometry staining.
CD4+CD25− T cells and CD4+CD25+ Treg cells sorting
Single cell suspension of the thymic glands of 7 days old mice were prepared, followed by sorting of the CD4+CD25− T cells and CD4+CD25+ Treg cells using the Miltenyi Biotec magnetic sorter (Miltenyi Biotec, Germany) and CD4+CD25+ T cell isolation kit (Miltenyi Biotec), following the manufacture's instruction.
In vitro stimulation
Sorted 5 × 104 CD4+CD25- or CD4+CD25+ T cells were cultured in 96-well flat bottom plates with RPMI 1640 (Sigma) supplemented with antibiotics. The cells were stimulated with plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml) for 3 days.
Flow cytometry
Thymus, PDLNs and spleen cells were stained with the following surface antibodies; FITC-conjugated anti-CD4 (RM4-5, eBioscience, San Diego, Carlifornia, USA), APC-H7-conjugated anti-CD8 (53–6.7, BD), BV605-, PE- or PB-conjugated anti-CD25 (PC61.5, Biolegend, San Diego, Carlifornia, USA or eBioscience), eFlour405-conjugated anti-CD44 (IM7, eBioscience) and APC- or PE-conjugated anti-Nrp1 (761705, R&D, Minneapolis, MN, USA). Then the cells were washed, fixed and permeabilized using the Mouse Regulatory T Cell Staining Kit #3 (eBioscience). The permeabilized cells were stained with APC- or PECy-7-conjugated Foxp3 (FJK -16s, eBioscience), APC- or PB-conjugated Helios (22F6, BioLegend), PE-conjugated IL-10 (JES5-16E3, BioLegend), PE-conjugated CTLA-4 (UC10-4B9, BioLegend), PE-conjugated Bcl-2 (BCL/10C4, BioLegend), PE-conjugated GITR (Biolegend) and PE-conjugated Ebi3 (355022, R&D). The stained cells were analysed on a BD LSRII Flow Cytometer (BD) at BioVis Uppsala University, Uppsala, Sweden. The data were analysed by using Diva 6.0 software (BD) or Flowlogic (eBioscience). Single stained and fluorescence minus one-stained controls were used for gating strategies.
Statistical analysis
The results were expressed as means ± SEM. SigmaPlot™ 12.3 software was used for the statistical analysis. Unpaired t-tests were used for comparisons between two groups. One-way ANOVA followed by Tukey's test were performed for multiple comparisons. A p-value of <0.05 was considered to be significant.
References
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat Rev Immunol 8, 523–532 (2008).
Sakaguchi, S., Wing, K., Onishi, Y., Prieto-Martin, P. & Yamaguchi, T. Regulatory T cells: how do they suppress immune responses? Int Immunol 21, 1105–1111 (2009).
Roncarolo, M. G. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 212, 28–50 (2006).
Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3, 135–142 (2002).
Von Herrath, M. G. & Harrison, L. C. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 3, 223–232 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immun 4, 337–342 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4, 330–336 (2003).
McHugh, R. S. et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16, 311–323 (2002).
Deiss, L. P., Feinstein, E., Berissi, H., Cohen, O. & Kimchi, A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes & Dev 9, 15–30 (1995).
Weiss, J. M. et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 209, 1723–1742, S1721, (2012).
Lechner, O. et al. Fingerprints of anergic T cells. Curr Biol: CB 11, 587–595 (2001).
Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, A. & Rudensky, A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol 3, 33–41 (2002).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184, 3433–3441 (2010).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 209, 1713–1722, S1711–1719 (2012).
Bruder, D. et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol 34, 623–630 (2004).
Milpied, P. et al. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol 39, 1466–1471 (2009).
Chia, R., Achilli, F., Festing, M. F. & Fisher, E. M. The origins and uses of mouse outbred stocks. Nat Genet 37, 1181–1186 (2005).
Gerberick, G. F., Cruse, L. W., Miller, C. M, Sikorski, E. E. & Ridder, G. M. Selective modulation of T cell memory markers CD62L and CD44 on murine draining lymph node cells following allergen and irritant treatment. Toxicol Appl Pharm 146, 1–10 (1997).
Lio, C. W. & Hsieh, C. S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Kolodkin, A. L. et al. Neuropilin is a semaphorin III receptor. Cell 90, 753–762 (1997).
Kolodkin, A. L., Matthes, D. J. & Goodman, C. S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 (1993).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Miyazaki, T. et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell 81, 223–231 (1995).
Mahmud, S. A. et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol 15, 473–481 (2014).
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol 15, 1070–1078 (2014).
Schliesser, U. et al. Generation of highly effective and stable murine alloreactive Treg cells by combined anti-CD4 mAb, TGF-beta and RA treatment. Eur J Immunol 43, 3291–3305 (2013).
Gottschalk, R. A., Corse, E. & Allison, J. P. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 188, 976–980 (2012).
Lathrop, S. K., Santacruz, N. A., Pham, D., Luo, J. & Hsieh, C. S. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med 205, 3105–3117 (2008).
Agus, D. B., Surh, C. D. & Sprent, J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med 173, 1039–1046 (1991).
Ayyoub, M., Raffin, C. & Valmori, D. Comment on “Helios+ and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans”. J Immunol 190, 4439–4440 (2013).
MacDonald, K. G. et al. Response to comment on “helios+ and helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans”. J Immunol 190, 4440–4441 (2013).
Akimova, T., Beier, U. H., Wang, L., Levine, M. H. & Hancock, W. W. Helios expression is a marker of T cell activation and proliferation. PloS one 6, e24226 (2011).
Daley, S. R, Hu, D. Y. & Goodnow, C. C. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med 210, 269–285 (2013).
Getnet, D. et al. A role for the transcription factor Helios in human CD4+CD25+ regulatory T cells. Mol Immunol 47, 1595–1600 (2010).
Neufeld, G. et al. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med 12, 13–19 (2002).
Sarris, M., Andersen, K. G., Randow, F., Mayr, L. & Betz, A. G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 (2008).
Thorvaldson, L., Holstad, M. & Sandler, S. Cytokine release by murine spleen cells following multiple low dose streptozotocin-induced diabetes and treatment with a TNFalpha transcriptional inhibitor. Int Immunopharm 3, 1609–1617 (2003).
Bennett, C. L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27, 20–21 (2001).
Acknowledgements
We are grateful to Drs. Dirk Pacholsky (BioVis, Uppsala University, Uppsala, Sweden) and Karin Gustafsson for valuable advice and discussion about flow cytometry analysis. We are also grateful to Livia Knörr for helping us with sorting the cells. This study was supported by the Swedish Research Council, Swedish Diabetes Association (Diabetesfonden) and the Ernfors Fund.
Author information
Authors and Affiliations
Contributions
K.S. designed the study, performed most of the experiments, analysed the data and wrote the manuscript. M.H. performed the experiments on NOD and C57B/6 mice together with K.S. L.T. and S.S. critically reviewed the manuscript and S.S. arranged funds for the study.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figures and Figure Legends
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Singh, K., Hjort, M., Thorvaldson, L. et al. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naïve mice. Sci Rep 5, 7767 (2015). https://doi.org/10.1038/srep07767
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07767
This article is cited by
-
Regulatory T cells-centered regulatory networks of skeletal muscle inflammation and regeneration
Cell & Bioscience (2022)
-
Rapid expansion of Treg cells protects from collateral colitis following a viral trigger
Nature Communications (2020)
-
Soluble glucocorticoid-induced tumor necrosis factor receptor regulates Helios expression in myasthenia gravis
Journal of Translational Medicine (2019)
-
Loss of TET2 and TET3 in regulatory T cells unleashes effector function
Nature Communications (2019)
-
Differential expression of Helios, Neuropilin-1 and FoxP3 in head and neck squamous cell carcinoma (HNSCC) patients
3 Biotech (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.