Abstract
Dopamine (DA) is a key neuromodulator in the brain that supports motor and cognitive functions. Here, we use apomorphine (Apo) and 3,3'-iminodipropionitrile (IDPN) to develop two rat models of Tourette's syndrome (TS), a common neuropsychiatric disorder characterized by stereotyped repetitive involuntary tics. The models enabled the assessment of unique ameliorative effects of Ningdong granule (NDG), a traditional Chinese medicine (TCM) preparation dedicated to the treatment of TS, on the striatal DA content of rats. By using high-performance liquid chromatography (HPLC), we found that long-term administration of NDG could, at least partially, restore the striatal dopamine alterations, either by increasing them after IDPN treatment or by decreasing them after Apo treatment. Taken together, our data indicated that NDG could ameliorate the abnormal striatal DA content dually and the unique therapeutic property may be meaningful for the treatment of TS.
Similar content being viewed by others
Introduction
Tourette's syndrome (TS) is a common chronic neurobehavioral and neuropsychiatric disorder characterized by stereotyped repetitive involuntary motor and phonic tics. Initial symptoms of TS usually start at childhood with a peak age between 7 to 15 years of age and persist to late adolescence or even early adulthood. Tics are more prevalent in males than in females with the ratio of 3–9:11. In some cases, tics can cause lifelong impairment and about 5% of TS patients have life-threatening symptoms including mild self-injurious behaviors and borderline personality disorder (BPD)2.
The fundamental pathophysiology of TS remains largely unknown, but a role for dysfunctions of dopaminergic system is suggested by high efficacy of dopamine D2 receptor (DRD2) antagonists3,4. Evidence from recent functional imaging studies and postmortem examinations suggest that increased D2 receptor density does play a key role in the pathogenesis of TS5,6. However, on the other hand, the role of DA in TS has never been clearly elucidated. Even though increased DA content has been proved in some pathological studies7,8. The increased DA level as well as the well-accepted increased density of D2 receptors are considered potent evidence in support of excessive dopaminergic activity as the potential pathogenesis of TS9. Other studies of the same kind have failed to find differences between TS patients and normal controls or have yielded conflicting results10,11. More interestingly, a number of studies have shown that DA agonists are also effective in suppressing tics12,13. These paradoxical findings suggest that, through other mechanism, the decreased striatal level of DA might also play a significant role in the pathogenesis of TS.
Apomorphine (Apo) and 3,3'-iminodipropionitrile (IDPN) are two manipulations used to develop TS animal models14,15,16,17. Previous published data revealed that both Apo and IDPN manipulated dyskinesia of TS models have demonstrated marked alterations in DA: intraperioneal injection of Apo to rats led to increased level of DA16, while rats exposed to IDPN demonstrated significant decreased alteration in DA content18,19,20. These TS models with contrary alterations in DA content might provide us new way to explore the multiple therapeutic value of anti-tics drugs on the neurotransmitter.
Ideal pharmacological treatment of TS are not presently available. The commonly used anti-tic drugs nowadays include alpha-adrenergic receptor agonist, typical neuroleptics and atypical neuroleptics. These agents, either agonist or antagonist of monoaminergic systems, are thought to act primarily by effecting their corresponding receptors thereby decreasing the output of the motor cortex. While the results are often highly variable and unfortunately associated with a series of side-effect burden. Haloperidol (Hal), a typical member of conventional neuroleptics, is thought to exert its clinical effects through striatal DRD24. Although clinical trials have shown its high potency in suppressing tics, side effects including sedation, electrocardiographic changes and extrapyramidal syndromes made Hal suboptimal in some patients4,21,22. The newer atypical neuroleptics are devoid of these side effects, however, they are associated with other side disorders including weight gain as well as diabetes4.
Despite the unfavorable limitations of the receptor interventionists, the neurotransmitters of these systems have far from been fully studied. In the current study, we focused on DA transmission in striatum, an essential component of basal ganglia which is fundamental for the appropriate sequencing of repetitive stereotypic movements23. As discussed above, striatal DA content, either in a increased or decreased manner, would play a role in the pathophysiology of TS. Perhaps, novel drugs with dual ameliorative effects on DA content will have therapeutic potential for TS.
Traditional Chinese medicine (TCM), developed and refined over the course of thousands of years, has been widely used in the prevention and treatment of various diseases in China, Japan, South Korea and other Asian countries24. Even though haven't been systematically studied, the benign ameliorative effects of TCM have long been widely accepted among native people in these countries. Ningdong granule (NDG), a compound preparation of TCM dedicated to the treatment of TS with the guidance of therapeutic principle of TCM, has been used as an anti-tic agent for years in clinics in China16,22,23,24,25,26,27. Our previous double-blind placebo-controlled trial has confirmed its clinical efficacy, with a reduction of 41.39% in tic severity and frequency reported26. Another open-label study has also suggested benefits of NDG in treating tics: of a total of 60 TS patients, up to 83.3% showed moderate to marked reductions in tic severity and frequency as assessed by the Yale Global Tic Severity Scale (YGTSS) and the long term (12 months) effective rate is 73.3% (44/60) compared with 25% (15/60) in the Hal-treated group31. No serious side effects were reported in both of the trials.
In further studies of the probable pharmacological mechanisms of NDG for treating TS, a dual ameliorative effect of NDG on the striatal DA content of TS rats came to our notice. Based on the aforementioned studies, we first developed two TS rat models to imitate different forms of dopaminergic dysfunctions: first, a TS rat model with increased striatal DA content; second, a TS rat model with decreased striatal DA content. Then, the potential dual values of NDG in reversing striatal DA content could be examined by high-performance liquid chromatography with electrochemical detection (HPLC-ECD). Additionally, we also investigated alterations in the stereotyped behaviors and correlated changes in DRD2 protein content were further determined by western blot analysis.
Methods
Preparation of NDG
The NDG formulation includes 4 different plant species, 3 animal substances and human placenta (Table 1). All of these formulations were provided by 999 Modern Chinese Medicine Co. Ltd. (999 Co. Ltd., Shenzhen, China) and prepared as previously reported19. After being dried, all of them were mixed in proportion and then macerated with distilled water for 1 h at room temperature and the whole mixture was decocted twice for 1 h each time. The filtrates were mixed and condensed and then dried by vacuum-drier at 60°C. The yield granule was stored at 4°C.
Experimental Animals and Behavior Recordings
All experimental procedures were carried out in compliance with relevant guidelines and regulations of the American Physiological Society. The protocols were also approved by the medical ethics committee of Provincial Hospital of Affiliated to Shandong University.
Wistar rats (male, 4 weeks old, 100 ± 20 g) were obtained from Laboratory Animal Center of Shandong University and they were housed in environmentally controlled quarters, temperature at 22 ± 2°C and humidity at 50 ± 10%, with a 12 h:12 h light/dark cycle (lights on 6:00–18:00). Laboratory rat diet and water were available ad libitum.
After a week acclimatization to their new quarters, a total of 70 rats were randomly assigned to control group (n = 10) and TS model group (n = 60). Rats in the control group received intraperitoneal infections of normal saline (NS) (0.9%) at a dose of 5 ml/kg/day and in the TS model group, two kinds of groups were contained: Apo group (n = 30) and IDPN group (n = 30). The former was intraperitoneally injected with Apo (Sigma Chemical Co., St. Louis, MO, USA) (2 mg/kg/day) while in the latter, the rats were injected with IDPN (Sigma Chemical Co., St. Louis, MO, USA) (150 mg/kg/day). Both Apo and IDPN groups were further assigned to 3 groups (10 rats per group) and the associated rats received intragastric administration with normal saline at 10 ml/kg/day (Apo + NS group and IDPN + NS group), NDG at 370 mg/kg/day (Apo + NDG group and IDPN + NDG group) and Hal (Shanghai Pharmaceutical Group Co. Ltd., Shanghai, China) at 1.0 mg/kg/day (Apo + Hal group and IDPN + Hal group) respectively for 8 consecutive weeks16,19.
On a weekly basis, the rats were rated for locomotor and stereotyped behaviors28 (Table 2) by two independent blind observers. Each animal was monitored for 1 min of every 5 min for 6 observation periods. The episodes accorded with the grades got the corresponding score and the average score were calculated based upon the data from observers as the objective indicator of behavioral alterations.
HPLC analysis
Regional concentration of DA was analyzed by high-performance liquid chromatography with HPLC-ECD (Waters 2465, Meliford, MA, USA) as previously described29. Briefly, the rats were sacrificed and the striatum tissues were dissected and immediately homogenized with 0.1 M perchloric acid, homogenates were centrifuged at 12,000 rpm for 15 min at 4°C and the resulting supernatants were stored at −80°C until analysis.
Before detection, the supernatants were collected and filtered through microcentrifuge filters. Compound separation was achieved on a C18 reverse-phase analytical column (50 mm × 2.1 mm, 1.9 µm particle size, Thermo, Rockford, IL, USA) with a mobile phase consisting of 150 mM citric acid, 150 mM trisodium citrate dihydrate, 100 mM ethylenediamine tetraacetic acid disodium salt (EDTA · 2Na), 1 mM sodium 1-heptanesulfonate and 10% methanol (v/v). Elution was carried out at a flow rate of 0.2 ml/min and the working electrode potential of the electrochemical detector was set at 0.8 V. The column was maintained at 28°C.
Western blot analysis
Rat striatum tissues were dissected and quickly homogenized on ice in RIPA lysis buffer (50 mM Tris-Hcl (PH 7.4), 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS)) containing protease inhibitors (PMSF) and centrifuged at 12,000 rpm for 15 min at 4°C. After determined by BCA protein assay kit (Comwin Biotech, Beijing, China), the protein amounts in the supernatant were diluted in 5 × loading buffer (Beyotime Inst Biotech, Haimen, China) and then boiled at 100°C for 5 min. SDS polyacrylamide gel (SDS-PAGE) electrophoresis was carried out on 10% Tris-glycine gels. The separated proteins were then electrophoretically transferred to PVDF membranes (0.45 µm Millipore, Bedford, MA, USA) that were treated previously with methanol and blocked with 1% BSA (Sigma Chemical Co., St. Louis, MO, USA) in TBS-T (Tris-buffered saline containing 0.1% Tween 20) for 1 hour at room temperature. After washing in TBS-T, the membranes were incubated with primary antibody against D2R antibody (1:1,000, Millipore, Chemicon, MA, USA) overnight at 4°C and then were further incubated with peroxidase-conjugated affinipure goat anti-rabbit antibody IgG (1:10,000, ZSGB-BIO, Beijing, China) for 1 hour at room temperature. Blots were developed with enhanced chemiluminescence detection system (Pierce, Rockford, IL) and analyzed semiquantitatively using the National Institutes of Health Image J program. To confirm equivalent loading of samples, the same membranes were incubated with anti-â-actin antibody (1:1,000, ZSGB-BIO, Beijing, China) and visualized via enhanced chemiluminescence as mentioned earlier.
Statistical analysis
Data are presented as mean ± SEM. A repeated measurements analysis of variance (ANOVA) was employed to evaluate the stereotypic behavior counts at different time points. Groups were compared using one-way ANOVA. All data were analyzed by the SPSS statistical software package (Version 17.0, SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered statistically significant.
Results
Assessment of stereotypic behaviors
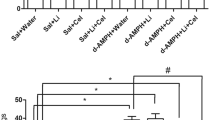
The effects of NDG on the stereotyped behaviors of the rats are shown in Figure 1. Using repeated measurements ANOVA, we found that both Apo and IDPN induced models had significant group effects (Apo: F2,27 = 22.73, p < 0.01; IDPN: F2,27 = 42.25, p < 0.01), indicating vary degrees of differences among Apo and IDPN groups. Compared to the same control rats shared by Apo and IDPN groups throughout the study, both of the pharmacological manipulations, Apo and IDPN, were associated with marked behavioral stereotypies in rats (Apo: p < 0.01; IDPN: p < 0.01). After being treated, in the rats exposed to Apo, long-term administration of NDG as well as Hal effectively rescued the Apo-induced stereotyped motor deficits (NDG: p < 0.01; Hal: p < 0.01) (Figure 1a). Meanwhile, in the rats subjected to IDPN, a steady reduction in stereotypies was also observed in stereotypies compared to the IDPN + NS group, after being treated by NDG and Hal respectively (NDG: p < 0.01; Hal: p < 0.01) (Figure 1b).
(a and b) Evaluations of stereotyped behaviors of experimental rats during an 8-week period. Data are expressed as the mean ± SEM. (n = 10 rats/group). The initial scores before treatments showed no differences among groups (p > 0.05). However, after 3 weeks of treatments, both NDG and Hal made a significant decrease in ethological recording scores compared with the TS model rats treated with NS (Apo + NDG group vs Apo + NS group, p < 0.01; Apo + Hal group vs Apo + NS group, p < 0.01; IDPN + NDG group vs IDPN + NS group, p < 0.01; IDPN + Hal group vs IDPN + NS group, p < 0.05), while there was no remarkable differences in score recording between these two treatments in either Apo or IDPN induced group. (Apo + NDG group vs Apo + Hal group, p > 0.05; IDPN + NDG group vs IDPN + Hal group, p > 0.05). a: #p < 0.05, significant difference between Apo + NDG group and Apo + NS group; *p < 0.05, significant difference between Apo + Hal group and Apo + NS group. b: #p < 0.05, significant difference between IDPN + NDG group and IDPN + NS group; *p < 0.05, significant difference between IDPN + Hal group and IDPN + NS group.
Effects of NDG on striatal DA concentration
The effects of NDG on the striatal DA content of the rats are shown in Figure 2. The ANOVA revealed significant effects for both groups (Apo: F3,36 = 30.79, p < 0.01; IDPN: F3,36 = 16.07, p < 0.01). Our data revealed that Apo and IDPN produced completely opposite effects on striatal DA content of the rats: manipulations of Apo significantly increased the DA concentration in striatum (p < 0.01), while the IDPN exposures were associated with marked decrease in DA content (p < 0.01), both compared with the same control rats. (Figure 2a)
(a and b) Effects of NDG on striatal DA content in experimental rats. Data are expressed as the mean ± SEM. (n = 10 rats/group). (a): Effects of NDG on striatal DA content in Apo-induced TS rats. #p < 0.01 represents when compared to the control group; *p < 0.01 represents when compared to the Apo + NS group. (b): Effects of NDG on striatal DA content in IDPN-induced TS rats. #p < 0.001 represents when compared to the control group; *p < 0.01, represents when compared to the IDPN + NS group.
As tested by HPLC, we found that, long-term administrations of NDG significantly reduced the up-regulated striatal DA content in Apo-induced rats (Apo + NDG group vs Apo + NS group p < 0.01) and increased the down-regulated striatal DA content in IDPN-induced rats (IDPN + NDG group vs IDPN + NS group p < 0.01). In contrast, Hal failed to produce any modification of striatal DA alterations in either Apo- or IDPN-treated rats (Apo + Hal group vs Apo + NS group, p > 0.05; IDPN + Hal group vs IDPN + NS group, p > 0.05). (Figure 2b)
Effects of NDG on DRD2 protein content in striatum
The effects of NDG on the striatal DRD2 protein content of the rats are shown in Figure 3. The ANOVA revealed a significant effect for groups (Apo: F3,36 = 24.69, p < 0.01; IDPN: F3,36 = 29.73, p < 0.01). Apo and IDPN exposures were associated with significantly increased DRD2 protein content in the striatum compared with the same control group (Apo: p < 0.01; IDPN: p < 0.01). After being treated, in the Apo groups, both NDG and Hal treatment prevented the Apo-induced elevation of DRD2 protein content in striatum (Apo + NDG group vs Apo + NS group p < 0.01; Apo + Hal group vs Apo + NS group p < 0.01) (Figure 3a). Meanwhile, results similar to Apo groups were also observed in the IDPN groups after being treated with NDG and Hal respectively (IDPN + NDG group vs IDPN + NS group p < 0.01; IDPN + Hal group vs IDPN + NS group p < 0.01) (Figure 3b).
(a and b) Effects of NDG on striatal DRD2 protein content in experimental rats. Data are expressed as the mean ± SEM. (n = 10 rats/group). (a): Effect of NDG on striatal DRD2 protein content in Apo-induced TS rats. #p < 0.01 represents when compared to the control group; *p < 0.01 represents when compared to the Apo + NS group; ##p < 0.01 represents when compared to the Apo + NS group. (b): Effects of NDG on striatal DRD2 protein content in IDPN-induced TS rats. #p < 0.01, represents when compared to the control group; *p < 0.01, represents when compared to IDPN + NS group; ##p < 0.01, represents when compared to the IDPN + NS group.
Discussion
From the unique theoretical and therapeutic system of TCM, people with TS usually suffer from insufficiency of yin in heart and liver30. According to the pathological changes, the ingredients of NDG were strictly chosen on the basis of the compatibility theory of TCM and TS patients would be treated for a particular nourishment of the yin in heart and liver27.
Our data showed that, similar to Hal, long-term administrations of NDG significantly mitigated the stereotyped abnormalities and reversed the up-regulated striatal DRD2 protein content in both Apo- and IDPN-treated TS rats. While in terms of striatal DA concentration, the effects of NDG and Hal are completely different. The present study demonstrated that, for the first time, long-term administrations of NDG could, at least partially, restore striatal DA alterations, either by increasing them after IDPN treatment or by decreasing them after Apo treatment. In contrast, Hal failed to produce any modification of the DA changes in either Apo or IDPN treated rats.
Based on achievements of previous investigators14,15,16,17, two pharmacological manipulations, Apo and IDPN, were selected to develop TS rat models. Early studies revealed that injection of dopaminergic agonist (e.g. Apo) to rodents led to behavioral stereotypies like licking, sniffing and biting, which could be blocked by DA antagonists16,31. Administration of Apo to healthy humans was also found to result in yawning and increase blinking32. Consistent with these findings, our data indicated that Apo exposure was associated with significant repetitive stereotyped movements of rats that could be attenuated by Hal treatment. Systemic administrations of IDPN to rats produced a dyskinetic-hyperkinetic syndrome characterized by choreiform movements and tic-like jerks of head and neck similar to that of TS for the life of animals33,34. In this study, as expected, marked stereotyped motor deficits were observed after IDPN injections and that could be reliably suppressed by Hal as previously described15,17.
Validity of animal models is evaluated on three levels: face, predictive and constructive14. A wide spectrum of abnormal movements have been classified as tics in animal models35,36 and the definition of complex motor tics has broadened tic-like movements in animals to include stereotypic behaviors35,37. Given these findings, the repetitive behavioral abnormalities developed by Apo and IDPN in our experiment could be related to TS. Predictive validity is usually dependent on the responses to neuroleptics and/or α2-adrenergic agonists, which are commonly used pharmacological treatments of TS13. In this study, both Apo and IDPN induced TS rats models responded well to Hal, achieved good predictive validity. Establishing construct validity is largely dependent on the strength of currently prevailing achievements or hypotheses concerning pathophysiology underlying human disorder. More than work on any other neurotransmitters, DA hypothesis still has greatly influenced our understanding of the etiology of TS. Apo, a direct dopaminergic agonist, has complex actions on striatal dopaminergic functions38. The present results confirmed and extended prior views that, exposed to Apo significantly increased DA concentration and DRD2 protein content in striatum. IDPN is known to interfere with several neurotransmitters including DA18,19,20 and evidence suggests that D2 receptors is also involved in the IDPN-induced dyskinesia18. Our data validated that IDPN exposure was associated with decreased DA as well as increased DRD2 protein content in striatum as compared to the saline-treated control rats.
To date, neuroleptics, among which Hal is commonly used, remain the cornerstone of TS treatment4. The efficacy of Hal is associated with its potency in blockage of DRD2 so as to decrease dopaminergic input to basal ganglia4,40. The basal ganglia is a brain area through which the cerebral cortex release desired behaviors and inhibit potentially unwanted ones. Blockage of D2 receptors removes dopaminergic suppression of basal ganglia and allows it to inhibit the undesired movements, however, that might otherwise interfere with the intended behaviors at the same time40.
With the unique dual ameliorative effects, NDG might have its superiority compared with the single-target pharmacological function of Hal. In this study, we concentrated on only two pathogenic forms of dopaminergic dysfunction, however, because of the highly heterogeneous nature of the human body there would be large differences in the pathophysiological changes among TS patients. The chief pathological changes of the TS patients who respond well to Hal might be the hyperfunction of dopaminergic system, while in some other patients, DA agonists have also been shown to suppress tics. Based on the prevailing theory of dysfunctional dopaminergic basal ganglia circuitry39, DA agonists acting at postsynaptic D2 receptors should facilitate tics rather than suppress them40. In light of these discrepancies, investigators have therefore postulated presynaptic inhibitory of DA receptors, which would impair DA release and sensitize the associated receptors40,41. The intriguing results of the clinical observations pointed out the fact that, the pathophysiology of TS may be more complicated than we expected. The involvement of multiple mechanisms of TS make it rather hard to get ideal responses with single-target anti-tic agents. While the unique properties of NDG in reversing the dopaminergic dysfunction caused by more than one mechanisms may have therapeutic potentials for treating these complex cases.
Although the goal of this study was not to examine TS with comorbidities, the IDPN-induced rat model, with the decreased striatal DA content as well as the increased DRD2 protein content, may provide some insight into a particular pathological form of TS: tics with the coexisting disorder of attention deficit hyperactivity disorder (ADHD). ADHD and obsessive-compulsive disorder (OCB) are the most common psychiatric comorbidities in TS42,43. The presence of comorbidities can add greatly to the complexity of the therapies whose treatments may conflict with the management of tics44,45. Consensus suggests that dopaminergic activity is lower than normal in children and adolescents with ADHD46,47, while excessive dopaminergic activity is still the most commonly accepted hypothesis for TS. Psychostimulants (e.g. methylphenidate and dextroamfetamine) are the most effective agents for uncomplicated ADHD, these drugs could be used with impunity in some individuals with TS, while in a few cases they could precipitate de-novo tics or aggravated pre-existing tics43. In these incompatible conditions, which are intractable to simple therapeutic principles, a single medication that could improve both ADHD and tics would be clinical useful. One of our previous open-label trials have proved the clinical efficacy of NDG in TS patients with ADHD with significant improvements in 25 of 30 TS children with ADHD, no serious side or toxic effect was reported48.
In summary, the present study has demonstrated that long-term NDG treatment significantly reversed stereotyped abnormalities and up-regulated DRD2 protein content in both Apo- and IDPN-treated TS rats. More importantly, long-term administration of NDG could make a dual reversion in striatal DA content from both increased and decreased level. In a word, the results of our data suggests that NDG is a potential therapeutic intervention for TS.
References
Bloch, M. H. & Leckman, J. F. Clinical course of Tourette's syndrome. J. Psychosom. Res. 67, 497–501 (2009).
Berthier, M. L., Campos, V. M., Kulisevsky, J. & Valero, J. A. Heroin and malignant coprolalia in Tourette's syndrome. J. Neuropsych. Clin. Neurosci. 15, 116–117 (2003).
Seignot, J. N. Un cas de maladie des tics de Gilles de la Tourette guéri par le R.1625. Ann. Méd-Psychol. 119, 578–579 (1961).
Hartmann, A. & Worbe, Y. Pharmacological treatment of Gilles de la Tourette syndrome. Neurosci. Biobehav. Rev. 37, 1157–1161 (2013).
Wolf, S. S. et al. Tourette syndrome: prediction of phenotypic variation in monozygotic twins by caudate nucleus D2 receptor binding. Science 273, 1225–1227 (1996).
Minzer, K., Lee, O., Hong, J. J. & Singer, H. S. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J. Neurol. Sci. 219, 55–61 (2004).
Wong, D. F. et al. Mechanisms of dopaminergic and serotonergic neurotrans-mission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacol 33, 1239–1251 (2008).
Steeves, T. D. et al. Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann. Neurol. 67, 170–181 (2010).
Singer, H. S., Rabins, P. & Coyle, J. T. Serum haloperidol levels in Gilles di la Tourette's syndrome. Biol. Psychiat. 16, 79–84 (1981).
Turjanski, N. et al. Pet Studies of the Presynaptic and Postsynaptic Dopaminergic System in Tourette's Syndrome. J. Neurol. Neurosur. Ps. 57, 688–692 (1994).
Stamenkovic, M. et al. No change in striatal dopamine re-uptake site density in psychotropic drug naive and in currently treated Tourette's disorder patients: a [(123)I]-beta-CIT SPECt-study. Eur Neuropsychopharmcol. 11, 69–74 (2001).
Cianchetti, C., Fratta, A., Pisano, T. & Minafra, L. Pergolide improvement in neuroleptic resistant Tourette cases: various mechanisms causing tics. Neurol. Sci. 26, 137–139 (1997).
Anca, M. H., Giladi, N. & Korczyn, A. D. Ropinirole for Tourette syndrome. Neurology 56, A121 (2001).
Bronfeld, M., Israelashvili, M. & Bar-Gad, I. Pharmacological animal models of Tourette syndrome. Neurosci. Biobehav. Rev. 37, 1101–1119 (2013).
Diamond, B. I., Reyes, M. G. & Borison, R. A new animal model for Tourette syndrome. Adv. Neurol. 35, 221–225 (1982).
Lv, H., Li, A., Ma, H., Liu, F. & Xu, H. Effects of Ningdong granule on the dopamine system of Tourette's syndrome rat models. J. Ethnapharmacol. 124, 488–492 (2009).
Wang, D., Li, W., Liu, X. et al. Chinese medicine formula ''Jian-Pi-Zhi-DongDecoction'' attenuates Tourette syndrome via downregulating the expression of dopamine transporter in mice. Evid. Based Complement Altern. Med. (2013).
Hirata, H., Ogawa, N., Asanuma, M. Effect of chronic ceruletide treatment on dopaminergic neurotransmitters, receptors and their mRNAs in the striatum of rats with dyskinesia induced by iminodipropionitrile. Brain Res. 604, 197–204 (1993).
Wakata, N., Araki, Y., Sugimoto, H., Iguchi, H. & Kinoshita, M. IDPN-induced monoamine and hydroxyl radical changes in the rat brain. Neurochem. Res. 25, 401–404 (1999).
Kawada, Y., Ogawa, N., Asanuma, M. & Mori, A. Neuropeptide levels in discrete brain regions in the iminodipropionitrile-induced persistent dyskinesia rat model. Regulatory Peptides 55, 103–110 (1995).
Shapiro, E. et al. Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette's syndrome. Arch. Gen. Psychiat. 46, 722–730 (1998).
Sallee, F. R., Nesbitt, L., Jackson, C., Sine, L. & Sethuraman, G. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette's disorder. Am. J. Psychiat. 154, 1057–1062 (1997).
Berridge, K. C. & Aldridge, J. Super-stereotypy I: Enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse 37, 194–204 (2000).
Chan, E., Tan, M., Xin, J., Sudarsanam, S. & Johnson, D. E. Interactions between traditional Chinese medicines and Western therapeutics. Curr. Opin. Drug Discov. Devel. 13, 50–65 (2010).
Li, A. Y., Cong, S., Lu, H., Li, J. J. & Zhao, L. Clinical observation on treatment of Tourette syndrome by integrative medicine. Chin. J. Integr. Med. 15, 261–265 (2009).
Zhao, L., Li, A. Y., Lv, H., Liu, F. Y. & Qi, F. H. Traditional Chinese medicine Ningdong gradule: the beneficial effects in Tourette's syndrome disorder. J. Int. Med. Res. 38, 169–175 (2010).
Li, A. Y. et al. Clinic research of Ningdong granule in treating Tourette syndrome. J. Shan. Uni. Trad. Chin. Med. 32, 33–35 (2008) (in Chinese).
Napier, T. C. & Istre, E. D. Methamphetamine-induced sensitization includes a functional up-regulation of ventral pallidal 5-HT2A/2C receptors. Synapse 62, 14–21 (2008).
Wei, L. L. et al. HPLC-ECD determination of on striatal extracellular levels of monoamine neurotransmitter. Chin. J. Pharmacol. Anal. 3, 467–470 (2011).
Li, J. J. An-yuan Li's experience of treatment for Tourette's disorder. J. Trad. Chin. Med. 50, 15–16 (2009) (in Chinese).
Randrup, A., Munkvad, I. & Udsen, P. Adrenergic mechanisms and amphetamine induced abnormal behaviour. Acta. Pharmacol. Toxicol. 20, 145–157 (1963).
Blin, O., Masson, G., Azulay, J. P., Fondarai, J. & Serratrice, G. Apomorphine-induced blinking and yawning in healthy volunteers. Brit. J. Clin. Pharmaco. 30, 769–773 (1990).
Cadet, J. L. The iminodipropionitrile (IDPN)-induced dyskinetic syndrome: Behavioral and biochemical pharmacology. Neurosci. Biobehav. Rev. 13, 39–45 (1989).
Al Kadasah, S. et al. Pentoxifylline attenuates iminodipropionitrile-induced behavioral abnormalities in rats. Behav. Pharmacol. 20, 356–360 (2009).
Crossman, A. R., Mitchell, I. J., Sambrook, M. A. & Jackson, A. Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. The site of drug action and a hypothesis for the neural mechanisms of chorea. Brain 111, 1211–1233 (1988).
McCairn, K. W., Bronfeld, M., Belelovsky, K. & Bar-Gad, I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 132, 2125–2138 (2009).
Barbeau, A. et al. Classification of extrapyramidal disorders. Proposal for an international classification and glossary of terms. J. Neurol. Sci. 51, 311–327 (1981).
Anden, N. E. et al. Evidence for dopamine receptor stimulation by apomorphine. J. Pharm. Pharmacol. 19, 627–628 (1967).
Robertson, M. M. & Stern, J. S. Tic disorders: new developments in Tourette syndrome and related disorders. Curr. Opin. Neurol. 11, 373–380 (1998).
Steeves, T. D. & Fox, S. H. Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog. Brain Res. 172, 495–507 (2008).
Farnebo, L. O., & Hamberger, B. Drug induced changes in the release of 3H-monoamines from field stimulated rat brain slices. Acta. Physiol. Scand. 37 (suppl), 35–41 (1971).
Cavanna, A. E., Servo, S., Monaco, F. & Roberson, M. M. The behavioral spectrum of Gilles de la Tourette syndrome. J. Neuropsychiat. Clin. Neurosci. 21, 13–23 (2009).
Leckman, J. F. Tourette's syndrome. Lancet 360, 1577–1586 (2002).
Gadow, K. D., Sverd, J., Sprafkin, J., Nolan, E. E. & Grossman, S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch. Gen. Psychiat. 56, 330–336 (1999).
McDougle, C. H. et al. The efficacy of fluvoxamine in obsessive-compulsive disorder: effects of comorbid chronic tic disorder. J. Clin. Psychopharm. 13, 354–358 (1993).
Levy, F. Synaptic gating and ADHD: a biological theory of comorbidity of ADHD and anxiety. Neuropsychopharmcol. 29, 1589–1596 (2004).
Iversen, S. D. & Iversen, L. L. Dopamine: 50 years in perspective. Trends Neurosci. 30, 188–193 (2007).
Tang, H. X. & Li, A. Y. Treatment of 30 cases of Tourette's syndrome with attention deficit hyperactivity disorder by “Ningdong Granule”. Sh. J. TCM. 47, 65–66 (2013) (in Chinese).
Acknowledgements
The article was supported by the National Natural Science Foundation of China (81273798), the Natural Science Foundation of Shandong province (ZR2012HM030), the Development Project of Science and Technology of Traditional Chinese Medicine of Shandong Province (2013ZDZK-085).
Author information
Authors and Affiliations
Contributions
F.Z. wrote the main manuscript and A.Y.L. made constructive proposals to the arrangements of the work. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, F., Li, A. Dual ameliorative effects of Ningdong granule on dopamine in rat models of Tourette's syndrome. Sci Rep 5, 7731 (2015). https://doi.org/10.1038/srep07731
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07731
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.