Abstract
Liquids in the Leidenfrost state were shown by Linke to self-propel if placed on ratchets. The vapour flow below the liquid rectified by the asymmetric teeth entrains levitating drops by viscosity. This effect is observed above the Leidenfrost temperature of the substrate, typically 200°C for water. Here we show that coating ratchets with super-hydrophobic microtextures extends quick self-propulsion down to a substrate temperature of 100°C, which exploits the persistence of Leidenfrost state with such coatings. Surprisingly, propulsion is even observed below 100°C, implying that levitation is not necessary to induce the motion. Finally, we model the drop velocity in this novel “cold regime” of self-propulsion.
Similar content being viewed by others
A drop of water levitates, if placed on a surface whose temperature is far above water's boiling point, as early described by Leidenfrost1. In such a situation, the liquid both evaporates and squeezes the underlying vapour cushion, so that the pressure arising from the vapour flow can support the drop. These soft hovercrafts are highly mobile, due to the absence of contact with their substrate. The conjunction of vapour production, no-adhesion and low friction was exploited by Linke et al., who showed that asymmetric teeth on the solid can propel the liquid levitating above (figure 1)2.
An asymmetric pattern can rectify the vapour flow, which creeps down the inclined teeth and the resulting viscous stress entrains the liquid2,3,4,5,6. Applications of this remarkable effect can be limited by the high temperature necessary for the motion2,3,4,5,6,7,8,9: the solid must be heated above its Leidenfrost temperature TL marking the transition to the levitating state, which consequently brings the liquid at its boiling point Tb (with Tb < TL). It is known that Leidenfrost temperature can be modulated by textures at the solid surface10,11. Fibrous microstructures, for example, largely increase TL12; conversely, superhydrophobic textures considerably lower the Leidenfrost temperature, as recently reported by Vakarelski et al.13 and del Cerro et al.14. On such materials, the transition between non-wetting and Leidenfrost states becomes continuous, owing to the repellent nature of the texture. We show here that drops of water can be propelled on superhydrophobic ratchets far below usual Leidenfrost temperatures and even below the boiling point of water, where adhesion remains low but evaporation still occurs. This significantly reduces both substrate and liquid temperatures, which greatly extends the parameter range where propulsion can be obtained.
Our experiments are performed using deionised water and brass substrates machined to achieve ratchets such as shown in figure 1 (wavelength λ = 1.5 mm and depth ε = 250 μm). Superhydrophobic coating is a colloidal solution, the Glaco Mirror Coat Zero purchased from Soft99 Co. Ratchets are drawn out of solutions of Glaco and dried at 150°C for half an hour. The process is repeated three times. With this coating at room temperature, water makes an advancing contact angle θa of 171 ± 2° and a receding angle θr of 165 ± 2°, with the characteristic low hysteresis (Δθ = θa − θr ≈ 6°) of such coatings. Solids are placed on heating plates and temperature is checked using a surface probe, with an accuracy of 3°C.
In order to characterize the Leidenfrost transition, the lifetime τ of water drops is measured as a function of ratchet temperature T. The drop, of initial volume Ω = 60 μL, is kept trapped by placing a brass ring coated with Glaco and brought at the same temperature as the ratchet. We compare in figure 2 the lifetime on ratchets either bare (red data) or microtextured (black data). (i) On bare brass, τ decreases from 200 s at 80°C to a few seconds between 100°C and 200°C (boiling regime); it suddenly jumps to 150 s above 200°C, which defines the Leidenfrost temperature TL of this substrate; at higher temperatures, it slowly decreases with T (Leidenfrost state). The same behaviour would be observed on flat brass. (ii) On a brass ratchet treated with Glaco, the function τ(T) is very different, as reported by Vakarelski et al. on a flat substrate13: the lifetime continuously decreases from 600 s at 80°C to 300 s at 100°C and keeps on decreasing at larger temperature until it meets the behaviour on bare brass above the Leidenfrost temperature TL on the latter substrate. The increase of lifetime below Tb = 100°C compared to bare brass (by a factor between 3 and 10) arises from the reduction of drop radius in a non-wetting state and from the presence of insulating vapour/air pockets in the microstructures, below the liquid. At larger temperatures, there is no sharp transition to the levitating state, suggesting a continuous transition between non-wetting (T ≤ Tb) and levitation (T ≥ Tb). We now wonder whether water remains propelled on superhydrophobic ratchets brought below 200°C, the minimum temperature necessary to induce self-propulsion on bare ratchets.
Lifetime τ of water drops of initial volume Ω = 60 μL, as a function of surface temperature T for two ratchets: bare brass (red data), or brass with a superhydrophobic Glaco coating (black data).
In both cases, the drop is maintained trapped inside a centimetre-size Glaco-coated ring placed on the ratchet.
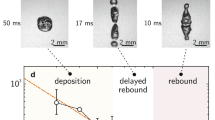
Horizontality of superhydrophobic ratchets is checked with an inspection spirit level (model 61 by Wyler AG Switzerland, precision of 10−4, that is, 100 μm/m) and their temperature T is varied between 75°C and 375°C. We deposit a water drop of volume Ω between 60 and 250 μL, corresponding to an equatorial radius R ranging from 2.9 to 5 mm. If the drop self-propels, we record its trajectory from the side with a fast camera, using backlighting to enhance the contrast. After a few centimetres, the drop position becomes linear in time, as seen in figure 3, corresponding to the stationary regime where the propelling force balances the friction experienced on the ratchet. The characteristic time of experiment (about 1s) being small compared to the 100 s of lifetime of the drop (figure 2), evaporation can be neglected along the motion and the drop radius of the drop considered as constant.
(a) Position x of water drops (of volume Ω = 60 μL) self-propelling on hot superhydrophobic ratchets, as a function of time t. Colours correspond to different ratchet temperatures (purple diamonds, T = 375°C; green squares, T = 243°C; red triangles, T = 125°C; blue circles, T = 85°C). (b) Side views of a water drop of 60 μL placed on a ratchet at T = 85°C. The bar shows 2 mm and 0.28 s separate two successive photos.
As seen in figure 3a, the drop velocity hardly depends on T at high temperature (green and purple data, corresponding to 243°C and 375°C, respectively). Contrasting with untreated ratchets, for which propulsion is observed above typically 200°C, the motion persists down to the liquid boiling point (red data), at a velocity smaller yet comparable (6.4 cm/s at 125°C, instead of 9 cm/s above 200°C). Hydrophobic microtextures shift the Leidenfrost point down to the boiling point of water and thus extend the range of propelling temperatures. More remarkably, motion still occurs below the boiling point: as seen in figure 3a (blue data) and figure 3b, a water drop self-propels at 2.4 cm/s on a superhydrophobic ratchet brought at 85°C. Hence the liquid does not need to levitate in order to move, which implies that motion can resist a contact with the substrate and that liquid can be below its boiling point (as confirmed in the supplemental material). Movie 1 summarizes these observations by comparing drop motions at various temperatures.
All the results can be collected by plotting the terminal velocity of drops as a function of the ratchet temperature T, as done in figure 4 for three different drop radii. Each point is an average on at least five measurements and the vertical error bars indicate the standard deviation. At high temperature (T > 250°C), velocity hardly depends on temperature or on drop radius, as observed on ratchets without superhydrophobic coating2. Below 200°C, the velocity falls and again seems independent of the drop radius. It eventually reaches 0 at a critical temperature Tc around 77°C.
Terminal velocity V of water on a superhydrophobic ratchet, as a function of the substrate temperature T, for three different drop radii R.
Propulsion is observed above a critical temperature Tc = 77°C significantly smaller than the boiling point Tb = 100°C. The dotted line shows Linke's regime at high temperature (T > 200°C) and the solid line represents Eq. 1.
The propulsion of Leidenfrost drops on ratchets arises from the rectification of the vapour flow by the asymmetric teeth, so that the levitating liquid is entrained by the vapour viscosity2,3,4,5. There is today no quantitative expression for the resulting driving force, in particular because of the complex geometry of the liquid interface beneath the drop. The situation might be simpler in the “cold regime of propulsion” described here, that is, below and around the liquid boiling point. Then we expect the vapour thickness to be fixed by the roughness on which the liquid sits. We denote h as the typical height of the microstructures, typically one micrometre for a Glaco treatment13. At the solid/liquid contact, we assume the saturation pressure Psat(T) in the vapour film (of order 100 kPa below the boiling point). This overpressure makes vapour flow inside the open network of microstructures. We suppose a flow geometry similar to the one observed when levitation is present. As shown experimentally and numerically4,5,6, propulsion arises from the succession of two perpendicular 2-D flows: down the teeth slopes first (which drags water above) and along the teeth base later (which permits the vapour evacuation). Here the flow down the teeth is made possible by the open nature of the microstructures and the detachment of liquid close to the teeth base (owing to surface tension) makes channels, along which vapour can be evacuated. At the scale h of microtextures, the lubrication equation holds, so that the mean vapour velocity U is given by Poiseuille's law: U ~ (h2/η) (Psat/λ) where η ≈ 2 10−5 Pa.s is the vapour viscosity. The corresponding viscous stress integrated over the drop surface area yields as a propelling force: F ~ (ηU/h) R2, so that we get: F(T) ~ hR2Psat(T)/λ. For millimetre-size drops on textured ratchets, this force is expected to be on the order of 10 μN (comparable to what is observed in the levitating state) and to increase with the temperature T.
In the cold regime considered here (T ≈ Tb, or below), the main effect opposing motion is liquid pining on the roughness tops, which is expressed by contact angle hysteresis. The corresponding sticking force scales as γRΔcosθ, denoting γ as the liquid surface tension. It is typically a few micronewtons for millimetre-size drops and for our measured hysteresis (Δθ ≈ 6°, Δcosθ ≈ 0.02). Hence the existence of a threshold Tc in temperature, as observed in figure 4 and expected to be given by the equation Psat(Tc) ≈ γΔcosθλ/Rh. Above Tc, the drop dynamics can be described by balancing the effective force Feff= F(T) −F(Tc) acting on the liquid with the dynamic friction opposing the motion. It was shown that this friction in a non-wetting situation is mainly inertial and caused by the soft shocks of water (of density ρ) as it hits the ratchet steps15. On each step, the corresponding force scales as ρV2Rε, which must be multiplied by the number R/λ of teeth below the liquid. Hence a friction force scaling as ρR2V2ε/λ, from which we can deduce the drop terminal velocity:

This velocity critically increases with the temperature above Tc and it is independent of the drop size, as indeed observed in figure 4. Its order of magnitude is 10 cm/s, again in agreement with the experiments. Eq. 1 is drawn in figure 4 with a solid line, taking for T the average between substrate and ambient temperatures and with a numerical coefficient of 0.4. It is found to fairly well describe the data and in particular to capture the high sensitivity towards temperature in this cold regime of propulsion. At high temperature, the Leidenfrost regime sets in and the description must be different, in particular because the vapour film thickness becomes a function of the temperature. On the one hand, a higher temperature implies a larger flux of vapour, favouring propulsion. On the other hand, it generates a thicker vapour film, so that the rectification of its flow is attenuated. These antagonistic effects qualitatively explain why the drop velocity in the levitating regime becomes quite insensitive to the substrate temperature and saturates around 10 cm/s, as early reported by Linke et al.2 and seen in figure 4 (dotted line).
We showed that drops of water propel on a superhydrophobic ratchet of temperature far below the usual Leidenfrost point and even below the water boiling point. This greatly extends the domain where ratchet propulsion can be used, which, together with the reduction of temperature inside the drop in this cold regime of propulsion, opens new fields of application for this device. Hence motion can be preserved despite solid/liquid contacts (at the tops of the microtexture on the ratchet surface), which shows that propulsion can be achieved with much thinner vapour films than assumed up to now. This might impact the size of the ratchet itself whose scale must compare to the vapour thickness, in order to efficiently rectify the vapour flow: with much thinner vapour films, it should be possible to observe drop propulsion on textured microratchets heated at moderate temperature.
References
Leidenfrost, J. G. De Aquae Communis Nonnullis Qualitatibus Tractatus (Ovennius, Duisburg, 1756).
Linke, H., Alemán, B. J., Melling, L. D., Taormina, M. J., Francis, M. J., Dow-Hygelund, C. C., Narayanan, V., Taylor, R. P. & Stout, A. Self-propelled Leidenfrost droplets. Phys. Rev. Lett. 96, 154502 (2006).
Ok, J. T., Lopez-Onna, E., Nikitopoulos, D. E., Wong, H. & Park, S. Propulsion of droplets on micro- and sub-micron ratchet surfaces in the Leidenfrost temperature regime. Microfluid. Nanofluid. 10, 1045–54 (2011).
Dupeux, G., Le Merrer, M., Lagubeau, G., Clanet, C., Hardt, S. & Quéré, D. Viscous mechanism for Leidenfrost propulsion on a ratchet. EPL 96, 58001 (2011).
Cousins, T. R., Goldstein, R. E., Jaworski, J. W. & Pesci, A. I. A ratchet trap for Leidenfrost drops. Journal of Fluid Mechanics 696, 215–227 (2012).
Baier, T., Dupeux, G., Herbert, S., Hardt, S. & Quéré, D. Propulsion mechanisms for Leidenfrost solids on ratchets. Phys. Rev. E 87, 021001 (2013).
Grounds, A., Still, R. & Takashina, K. Enhanced droplet control by transition boiling. Sci. Rep. 2, 720 (2012).
Hashmi, A., Xu, Y., Coder, B., Osborne, P. A., Spafford, J., Michael, G. E., Yu, G. & Xu, J. Leidenfrost levitation: beyond droplets. Sci. Rep. 2, 797 (2012).
Marin, A. G., Arnaldo del Cerro, D., Romer, G. R. B. E., Pathiraj, B., Huis in 't Veld, A. & Lohse, D. Capillary droplets on Leidenfrost micro-ratchets. Phys. Fluids 24, 122001 (2012).
Bernardin, J. D. & Mudawar, I. The Leidenfrost point: experimental study and assessment of existing models. J. Heat Trans. 121, 894–903 (1999).
Feng, R., Zhao, W., Wua, X. & Xue, Q. Ratchet composite thin film for low-temperature self-propelled Leidenfrost droplet. J. Coll. Int. Sci. 367, 450–454 (2012).
Weickgenannt, C. M., Zhang, Y., Sinha-Ray, S., Roisman, I. V., Gambaryan-Roisman, T. et al. Inverse-Leidenfrost phenomenon on nanofiber mats on hot surfaces. Phys. Rev. E 84, 036310 (2011).
Vakarelski, I. U., Patankar, N. A., Marston, J. O., Chan, D. Y. C. & Thoroddsen, S. T. Stabilization of Leidenfrost vapour layer by textured superhydrophobic surfaces. Nature 489, 274–277 (2012).
del Cerro, D. A., Marín, A. G., Römer, G. R. B. E., Pathiraj, B., Lohse, D. & Huis in 't Veld, A. J. Leidenfrost Point Reduction on Micropatterned Metallic Surfaces. Langmuir 28, 15106–15110 (2012).
Dupeux, G., Le Merrer, M., Clanet, C. & Quéré, D. Trapping Leidenfrost drops with crenelations. Phys. Rev. Lett. 107, 114503 (2011).
Author information
Authors and Affiliations
Contributions
G.D., P.B. and Q.M. did the experiments and C.C. and D.Q. wrote the main manuscript text. All authors designed the experiments and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Drop temperature
Supplementary Information
Self-propulsion of water on a hot superhydrophobic ratchet
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Dupeux, G., Bourrianne, P., Magdelaine, Q. et al. Propulsion on a superhydrophobic ratchet. Sci Rep 4, 5280 (2014). https://doi.org/10.1038/srep05280
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05280
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.