Abstract
In this article, we present a new paradigm for organometallic hybrid perovskite solar cell using NiO inorganic metal oxide nanocrystalline as p-type electrode material and realized the first mesoscopic NiO/perovskite/[6,6]-phenyl C61-butyric acid methyl ester (PC61BM) heterojunction photovoltaic device. The photo-induced transient absorption spectroscopy results verified that the architecture is an effective p-type sensitized junction, which is the first inorganic p-type, metal oxide contact material for perovskite-based solar cell. Power conversion efficiency of 9.51% was achieved under AM 1.5 G illumination, which significantly surpassed the reported conventional p-type dye-sensitized solar cells. The replacement of the organic hole transport materials by a p-type metal oxide has the advantages to provide robust device architecture for further development of all-inorganic perovskite-based thin-film solar cells and tandem photovoltaics.
Similar content being viewed by others
Introduction
Organometallic perovskite materials have been studied intensively since the pioneer work delivered by Kojima et al. which demonstrated that the organic-inorganic hybrid perovskite behaved like a light harvester1. Successive report by Park's group showed improved performance based on the similar liquid dye-solar-cell like device2. Until the breakthrough by the introduction of solid organic hole conductor to replace the liquid electrolyte, the new perovskite-type solar cells achieved remarkable efficiency and attracted enormous attention3,4. Since then, massive research works using various contact materials and device configurations, evolved from dye-sensitized solar cells (DSCs) with mesocsopic or super-mesoscopic structure and extreme thin absorber (ETA) structure, to organic photovoltaics (OPVs) with a planar heterojunction (PHJ), have emerged during the past few years5,6,7,8,9,10. Recent progresses with 15% power conversion efficiency (PCE) in either a mesoscopic or a PHJ have demonstrated that this organic-inorganic hybrid perovskite material is one of the most promising candidates for cost effective solar absorber in the future11,12,13. Efficiency exceeding 20% might be realized by further optimization through fill factor improvement and tandem architecture with multiple solar cells6,14. The beauty of perovskite material is the diversity in its device architectures due to the distinct material properties such as broad absorption spectra range with high extinction coefficient, ambipolar diffusion and long carrier diffusion length15,16,17. These merits allow the organic-inorganic hybrid perovskite being a light absorber superior to those of the solution-based photovoltaic such as DSCs, OPVs or other thin-film photovoltaics.
In addition, the voltage output of perovskite-based solar cells is relatively high among the other solution-processed photovoltaics mainly due to the minimized intrinsic potential losses that are inevitable for interfacial charge transfer in DSCs and the cost of exciton dissociation energy in OPVs. Open-circuit voltage (Voc) more than 1 volt, which is close to the inherent thermodynamic limit, has been demonstrated in many reports8,12,13,18,19. Currently the most successful device configuration is the junction (either mesoscopic or PHJ) with a n-type metal oxide/perovskite/hole transport materials (HTMs) where the metal oxide layer and the organic HTM act as selective contacts to facilitate charge extraction11,12,13,20,21. Another inverse structure, using non-oxide n-type material as electron acceptor composed of poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate)(PEDOT:PSS)/perovskite/fullerene planar junction, has been demonstrated with great success and remarkable conversion efficiency recently7,8,9,22. This OPV-like sandwich bilyaer junction structure has great potential for low temperature process and flexible devices8,13,22. A basic photovoltaic device is the sandwich of light absorbing materials between effective selective contacts. For perovskite absorber, the most commonly used n-contacts are TiO23,11,12,23,24,25,26, ZnO13,19,27, fullerene derivatives7,8,9 while the successful p-contacts are mostly organic materials such as 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamino)-9,9′- spirobifluorene (Spiro-OMeTAD) or other hole transporting materials (like poly-3-hexylthiophene (P3HT) or poly-triarylamine derivative)18,22,28,29,30,31,32,33. Recently, perovskite-based cell using CuI inorganic p-type semiconductor as hole transport layer delivered 6% efficiency has been reported34. Mainly due to the unfavorable energy level match between the valence band of CuI and perovskite, the voltage was considerably reduced compared to most of the highly efficient perovskite solar cells. However, this research highlighted the direction to develop all-inorganic materials for perovskite-based photovoltaic devices.
NiO has been one of the rarely successful candidates as p-type contact material for OPVs and DSCs35,36,37,38,39,40,41,42. Since organometallic perovskite has showed amibipoar charge transport behavior, sensitization of p-type wide band gap oxide shall be a feasible approach as the conventional n-type sensitization concept. Thus, we constructed the heterojunction solar cells with a p-i-n active layer composed of the mesoscopic NiO/peroskite/[6,6]-phenyl C61-butyric acid methyl ester (PC61BM) structure. In this report, we realized a highly efficient (9.51%) mesoscopic NiO/perovskite junction where the p-contact is replaced with mesoscopic inorganic metal oxide material to feature efficient charge separation in the NiO/perovskite interface. The success of efficient p-type sensitization or heterojunction solar cells is critical for the future design of tandem devices or p-n dual-sensitization configuration in the future36.
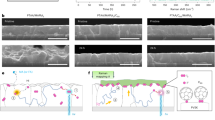
The details for the experimental procedures are described in the Methods section. The images of devices after NiOx thin film, NiO nanocrystalline (NiOnc) and perovskite deposition are illustrated in Fig. 1(a). The substrate coated with NiOx/NiOnc remained optically transparent with certain absorption in the range of 400 ~ 450 nm (See Supplementary Fig. 1S for transmission spectra). After the perovskite is deployed into the mesoscopic electrode, the device turned dark brown as shown in the right photo on Fig. 1(a). The full device structure is shown in Fig. 1(b) with the energy level of the each material illustrated in Fig. 1(c). The energy level of NiOx was taken from our previous study which the valance band was determined by ultraviolet photoelectron spectroscopy (UPS)43. The energy level for the NiO nanoparticle was given from the literature reported by Powar et al. where the valence band edge of NiO was measured using photoelectron spectroscopy in air (PESA)37. The ideal energy levels of the lowest unoccupied molecular orbital (LUMO) of PC61BM and valence band edge of NiO are located in the vicinity of the band edges of perovskite. This arrangement is appropriate for receiving high voltage with minimum loss providing the charge separation process between the absorber and selective contacts is efficient. The scanning electron microscopy (SEM) images of the corresponding illustrations in Fig. 1(a) are shown in Fig. 2. The NiOx thin film is very compact without pinholes or crevices as seen in Fig. 2(a). In Fig. 2(b), the NiO nanocrystalline showed feature sizes around few tenths of nanometers. Some aggregates above 100 nm are observed due to incomplete dispersion. We perceived Fig. 2(c) the filling of perovskite nanocrystals on the mesoscopic porous NiOnc network after sequential deposition of PbI2 and CH3NH3I with relative conformal coating leaving some small pores between the particles. It seems that no thick perovskite overlayer is formed on top of the NiOnc porous film, which suggests the role of PC61BM and bathocuproine (BCP) coating is critical for making good contact with the subsequent metal electrode. The cross sectional SEM image of the full device is presented in Fig. 2(d) showing the active mesoscopic NiOnc/perovskite layer is around 250 nm.
The current-voltage characteristics are presented in Fig. 3(a) with their corresponding incident photon to electron conversion efficiency (IPCE) in Fig. 3(b) and photovoltaic parameters listed in Table 1. The best-performing cell using PC61BM delivered high Voc of 1040 mV with short-circuit photocurrent density (Jsc) of 13.24 mA/cm2 and 69% fill factor, leading to an overall PCE of 9.51%. Another device using [6,6]-phenyl C71-butyric acid methyl ester (PC71BM) obtained very similar photovoltaic performance with Voc of 1000 mV, Jsc of 14.65 mA/cm2 and fill factor of 64%, resulted in an overall PCE of 9.44%. The effects of these two fullerene derivatives on their photovoltaic performances are very similar to what previously reported in OPV, which the device composed of PC71BM acceptor has slightly blue-shifted IPCE with lower voltage and higher current characteristics44,45,46,47. Whether the absorption of PC71BM between 400 ~ 600 nm has contribution to the photocurrent remained unclear and further investigations are in progress to understand this phenomenon. However, the thickness of the PC61BM or PC71BM layer was extremely thin (20 ~ 25 nm) in our device so that the differences in current response is relatively minor compared to those of OPVs. The cut-off of the IPCE below 450 nm is due to the absorption of NiOx/NiOnc which filtered the transmission of incident light35. Transmission of indium tin oxide (ITO glass) coated with NiOx/NiOnc is given in supplemental information (Fig. S1). The high Voc received from the junction demonstrated the advantage of energy level alignment between light absorber and the selective contacts. This extraordinary voltage output is a distinct characteristic for all the highly efficient perovskite-based photovoltaic devices11,12,13,22. The energy loss in charge extraction is minimized by using PC61BM whose LUMO is nearly identical with the conduction band edge of perovskite while the NiOnc valance band is close to that of perovskite.
(a) The current-voltage characteristics of photovoltaic devices made of mesoscopic NiOnc/perovskite/PCBM hetrojunction solar cell (red line for PC71BM and blue line for PC61BM) and planar NiOx/perovskite/PC61BM heterojunction solar cells (green line). (b) The IPCE of mesoscopic NiOnc/perovskite/PC71BM device (red line), mesoscopic NiO/perovskite/PC61BM device (blue line) and planar NiOx/perovskite/PC61BM heterojunction solar cell (green line). (C) The light harvesting efficiency (LHE) and absorbance of mesoscopic NiOnc/perovskite film (blue dot and blue line) and planar NiOx/perovskite film (green dot and green line).
To elucidate the effect of the mesoscopic structure, a flat electrode of oxide ITO/NiOx (without the NiO nanocrystalline layer) was applied for comparison. The flat junction cell delivered PCE of 7.40% (green line in Fig. 3 (a)) which is consistent with our previous report on the PHJ perovskite/fullerene solar cells43. The implement of mesoscopic NiO layer provided an increased film thickness to host the light absorbing perovskite material and prevented the risk of morphological defect that deteriorate the photovoltaic function. The improved photovoltaic performances of the mesoscopic devices are obvious and mainly due to the slightly increased current and voltage with respect to the PHJ cell. We attributed this phenomenon to the better morphological formation of perovskite in mesoscopic electrode compared with that of NiOx. In our previous work, we have found that the Voc for the PHJ devices is influenced by the work function of electrode interlayer, the spin speed of perovskite coating and surface morphology43. The SEM images of perovskite coated with various deposition conditions on the planar NiOx are available in literature43. One image of the perovskite thin film spin coated with 9500 rpm on NiOx for the PHJ device applied in this article is presented in Figure S2. Some small voids are presented on the surface which could impair the photovoltaic performance (mainly Voc) due to internal shunt between the selective contact thin film in the PHJ device. When a mesoscopic electrode is applied as charge (hole) collector, it is expected that the sequential deposition method provides better filling of perovskite into the nano-porous pore and more conformal surface coverage on the NiOnc surface which prevent direct contact between the n-contact layer (PCBM) with the p-contact materials (NiOnc or NiOx) This thin overlayer capping of perovskite on the NiOnc is advantageous for the voltage. Another difference between mesoscopic and PHJ devices is the amount of perovskite loading on the substrate ending up with more light harvesting efficiency (LHE) in the mesoscopic junction device than for the PHJ device. The measured absorbance and calculated LHE (LHE = 1-10−A) are presented in Fig. 3 (c) where we find a nearly saturated LHE for the layer of perovskite coated on the mesoscopic NiOnc thin film. The transmission loss is not significant in the PHJ device without the presence of NOnc layer as we can observe from the IPCE in Fig. 3(b) where the quantum conversion efficiency at the short wavelength region (400 ~ 500 nm) remained high. The IPCE of the PHJ device basically follows the LHE curve shown in Fig. 3(c). On the other hand, the mesoscopic device received enhanced IPCE on the long wavelength due to the improved optical density from perovskite loading. In the actual device, the reflection of the back electrode (Al in this case) has to be taken into account for the optical process in the photoactive layer. For both the PHJ and the mesoscopic devices, the transmitted flux in the red part will be scattered back into the absorber thus resulted in an increased optical path and improved IPCE. This is the reason that the IPCE of PHJ is higher than the LHE in the long wavelength region. Meanwhile, we do observe an increased IPCE response in the red part for the mesoscopic cells compared with the PHJ cells7,43. From the LHE in Fig. 3(c) and the cross sectional SEM image shown in Fig. 2(d), the mesoscopic junction is about 250 nm which is thick enough to absorb 90% of the incident light. The IPCE of these two devices seem to be lower than the state-of-the-art devices. We believe that there are still some charge collection losses in the NiO/perovskite mesoscopic junction. The fill factor of 64 ~ 69% has not yet reached an optimized value. Further improvements on the material quality of NiO with better crystallinity, smaller particle size and well-dispersed mesoporous nanostructure are expected to promote the overall photovoltaic performance (especially the current) of this p-type oxide/perovskite heterojunction device. Using chloride-containing perovskite (CH3NH3PbI3-xClx) that has been reported with longer carrier diffusion length might be another approach to achieve higher efficiency15. Concerning the reproducibility for this type of device, statistic histogram on the photovoltaic parameters for 62 devices are summarized in supplemental information Fig. S3.
The key question for this newly developed NiO/perovskite junction is whether the holes in the photoexcited perovskite transfer into the NiO particles or the mesoscopic network only acts as a scaffold like the Al2O3 meso-superstructured solar cell4. Our previous photoluminescence (PL) spectra results demonstrated that NiOx is capable of quenching the PL of perovskite43. To scrutinize the quenching process of perovskite by NiOnc, the PL spectrum of NiOnc/perovskite thin film used in the current study is shown in Fig. 4(a) in comparison with a reference of mesoscopic Al2O3 nanoparticles (Al2O3(nc))/perovskite thin film. We observed strong quenching prevailed with the NiOnc/perovskite sample, which implied charge transfer occurred at the NiOnc/perovskite interface. PL has been applied to examine the quenching of the perovskite in junction with various contact materials8. Recent results on quenching of PL in the composite perovskite films showed that PCBM is a very efficient n-type quencher while Spiro-OMeTAD, PEDOT:PSS, V2O5, NiO and P3HT are efficient p-type counterparts8,28. The NiO/perovskite film was observed with a very high yield (95%) of PL quenching but a well-performed device was not developed due to poor surface coverage and morphological control for the formation of uniform perovskite layer on NiOx film8. Our approach of using a mesoscopic NiOnc layer provided a mesoporous matrix for sufficient loading of the perovskite material and lowered the demand for control of morphology as for the PHJ. To further confirm the carrier separation phenomenon, we performed the photo-induced transient absorption (PIA) measurement to examine the signature of long-lived charge separation species between various junctions. PIA spectra of the samples containing film configurations of G/P, G/Al2O3/P, G/NiO/P and G/NiO/P/PC61BM (G stands for glass and P for perovskite) were carried out in the spectral range between 600 ~ 1500 nm at the excitation wavelength of 460 nm and the results are shown in Fig. 4(b). The PIA spectra of the G/P (brown) and G/Al2O3/P (purple) films show only the negative signals in the spectral region below 800 nm representing the characteristics of the absorption and emission of the perovskite species. In contrast, the PIA spectra of the G/NiO/P (blue for NiOx/NiOnc and green for NiOx substrate) film show a broad spectral feature above 800 nm which we assigned to the long-lived charge-separation state of NiO+/P−. The PIA signal in the NiOx/NiO(nc) film was much greater than that in the NiOx/P film indicating that the NiO+ species was produced in a larger extent for the former than for the latter. The oxidized NiO species has been observed in the electrochemical spectrum of nanostructured NiO featuring a broad range of absorption in the infrared region which corresponds to our PIA feature48. For the G/NiO/P/PC61BM (red) film containing the electron-accepting layer, the PIA spectrum was quite similar to that of G/NiO/P film in the spectral range 900 ~ 1500 nm. Since PCBM has been verified to be an effective electron acceptor in perovskite/PCBM junction8,9,15,16,22,49,50, the above evidence confirms that the observed broad absorption band is mainly due to the formation of the NiO+ cationic species upon photo excitation of the perovskite/NiO film at 460 nm. The PIA results revealed that the charge separation at the interface between perovskite and NiO is viable so that the resulting perovskite in our device is sandwiched between two efficient selective contacts that facilitate charge extraction featuring very high VOC and great device performance. Further investigations on ultrafast relaxation and carrier dynamics in the NiO/perovskite interface are underway to understand the underlying charge transport kinetics governing the operation of this type of solar cells.
The photoluminescence (PL) spectra (a) and photoinduced transient absorption spectra (b) of perovskite coated on various substrates.
In (a), the red line presents the PL of perovskite coated on mesoscopic Al2O3 nanoparticles and the blue line of perovskite coated on NiOnc. In (b), NiOx denotes the planar spin coated thin film, NiOnc means the mesoscopic NiO nanocrystalline thin film and Al2O3(nc) refers to mesoscopic Al2O3 nanocrystalline thin film.
Many efforts have been contributed to improve the device performance for p-type sensitized solar cells. The state-of-the-art p-type DSCs delivered less than 1.5% PCE37,38,40. The reasons for this much lower photovoltaic action compared with its n-type sensitization counterpart have been ascribed to the fast recombination, short hole diffusion length in p-type semiconductor and unfavorable redox energy levels of electrolyte with respect to the p-type metal oxide valence band40,51. The performance of the solar device described in this article greatly outperformed the conventional p-type sensitized solar cells or other p-type oxide semiconductor heterojunction solar cells. The success of such highly efficient p-type metal oxide heterojunction solar cells is vital for the future development of fully inorganic perovskite-based solar cells as well as the tandem devices.
Our results strongly confirmed the functionality of p-type NiO metal oxide acting as a selective contact is feasible. The future perspective is to integrate n-type contact material for the construction of a fully inorganic p-i-n heterojunction solar cell with perovskite absorber sandwiched between two wide band gap oxide semiconductors. This offers a configuration of superior thin film (or super mesoscopic) photovoltaic device using robust and extreme thin photoactive material. Additionally, our device provided a base for the tandem architecture or p-n dual sensitization photovoltaic. It would be crucial to explore low temperature process for metal oxide deposition and to introduce interconnection transparent connection layer for tandem cells. These topics are currently the subjects of research focuses in our group.
In conclusion, a methylammonium lead iodide (CH3NH3PbI3) perovskite/NiO heterojunction solar cell is developed to achieve remarkable efficiency of 9.51% of power conversion efficiency. This is the first demonstration of a p-type mesoscopic metal oxide as an efficient hole collecting contact. The success of this heterojunction will provide varieties for cell design and the realization of fully inorganic mesoscopic solar cells since the organic p-contact widely used in the past can now be replaced by the robust wide band gap metal oxide reported herein. It is expected that the device stability could be improved significantly with the application of metal oxide selective contact materials. We believe that further efforts can be made on the material optimization and judicious selection of contact materials and robust device or tandem configuration could be achieved for perovskite-based solar cells.
Methods
Fabrication of NiOx solution
The precursor for NiOx film coating was prepared with 0.5 M nickel formate dihydrate (Alfa Aesar) in ethylene glycol solution containing 1 M ethylenediamine (Aldrich) and filtered with 0.45 μm nylon filters41.
Fabrication of NiOnc paste
The mesoporous NiO solution used for spin coating was prepared by diluting slurry NiO with anhydrous ethanol in a ratio of 1:7. Slurry NiO was prepared by mixing 3 g of NiO nanopowder (Inframat) in 80 ml ethanol and subsequently adding with 15 g of 10 wt% ethyl cellulose (in EtOH) and 10 g of terpineol. The solution was stirred and dispersed with ultrasonic horn and concentrated with rotary evaporator for ethanol removal until 23 mbar.
Fabrication of CH3NH3I
CH3NH3I was synthesized as our previous literature. Methylamine (CH3NH2) (13.5 mL, 40 wt% in aqueous solution, Alfa Aesar) and hydroiodic acid (HI) (15.0 mL, 57 wt% in water, Alfa Aesar) were stirred at 0°C under nitrogen atmosphere for 2 h. After the reaction, the solvent of the solution was evaporated using a rotary evaporator. A white powder, methyl ammonium iodide (CH3NH3I), was generated by the reaction. The precipitate was washed with diethyl ether (Sigma–Aldrich) three times and dried at 60°C in a vacuum oven overnight1,3,7,25.
Device fabrication
The device fabrication was similar to our previous published procedure7,43. Patterned ITO-coated glass substrates (Ritek Corp., 15 Ω/sq.) are ultrasonically cleaned with soap water, deionized water and ethanol. Then, the substrates are rinsed with 2-propanol and Acetone, followed by UV-Ozone cleaner (Model: 42, Jelight, USA) treatment for 25 minutes. NiOx electrode-interlayer, using solution synthesized as described previously, was spun-cast onto the substrates at 4000 rpm for 90 s using spin-coater and then annealed at 300°C for 1 hour. After NiOx film was cooled to room temperature, the substrates are spin-coated with NiO(nc) at 4000 rpm for 30 seconds. After being annealed at 400°C for 30 minutes, the resulting films will form mesoporous NiO film. CH3NH3PbI3 is deposited on NiOx by one step spin coating43 (9500 rpm for PHJ device) or sequential deposition method on mesoporous NiOnc film (mesoscopic devices) with procedures referred to reported literature11. In order to have PbI2 solution (1 M in N,N-dimethylformamide) uniformly deposited well in the mesoscopic pore, the substrates are preheated at 70°C prior to the deposition of PbI2. PbI2 solution is spun onto the mesoporous NiOnc film at 6500 rpm for 5 seconds, followed by annealing at 70°C for 30 minutes. After cooling to room temperature, the resulting films are immersed into 2-propanol to pre-wet the substrates and then dipped into a solution of CH3NH3I (10 mg/ml in 2-propanol) for 40 seconds at room temperature and then annealed at 70°C for 30 minutes. The color of the resulting films becomes dark brown from bright yellow. PC61BM or PC71BM (20 ~ 25 nm) (> 99%, Solenne, Netherlands), BCP (10 nm) (Aldrich) and Al (100 nm) were thermally deposited on the substrate inside a vacuum chamber (10−6 Torr). The deposition thickness was monitored by the quartz crystal balance. The active area of the device is 0.06 cm2. All the procedures are implemented inside a nitrogen-filled glove box with oxygen and moisture levels < 1 ppm except for fabrication of NiOx electrode-interlayer and mesoporous NiOnc film.
Absorbance and Light Harvesting Efficiency (LHE)
The UV-vis spectra were measured by a spectrometer (U-4100, Hitachi). The samples for the optical characterization of perovskite are deposited on ITO/NiOx (spin coated at 9500 rpm) and ITO/NiOx/NiOnc (sequential deposition) substrates for planar and mesoscopic thin film respectively. The substrates are taken as baseline for the measurements.
Photoluminescence spectroscopy
Room-temperature micro-photoluminescence measurements were performed using the He–Cd laser at 325 nm with optical power of 7 mW. A NUV 40× objective lens was used for the collection of the pump light and the emitted light from the sample. The emission was dispersed by a 0.32 m focal length spectrometer with 1800 grooves/mm and detected by Liquid N2-cooled charged couple device (CCD) detector.
Photo-induced transient absorption
Excitation of the thin-film samples was achieved by a cw laser at 460 nm and modulated with a chopper (Thorlab, MC2000) at 19 Hz. The light source of the absorption spectra was provided by a Xenon lamp (PTi A-1010, 150 W) coupled with a monochromator (Acton Research Corporation, SpectraPro-300i). The PIA signals were obtained with a lock-in amplifier (Stanford Research System, SR830) via two detectors – Si photodiode (Thorlab, DET100) in the visible/NIR region (600–900 nm) and Ge detector (Thorlab, DET50B) in the NIR region (800–1500 nm). All the PIA measurements were performed right after the samples were prepared.
References
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Im, J.-H., Lee, C.-R., Lee, J.-W., Park, S.-W. & Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3, 4088–4093 (2011).
Kim, H.-S. et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2, 591 (2012).
Lee, M. M., Teuscher, J., Miyasaka, T., Murakami, T. N. & Snaith, H. J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 338, 643–647 (2012).
Noh, J. H., Im, S. H., Heo, J. H., Mandal, T. N. & Seok, S. I. Chemical Management for Colorful, Efficient and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 13, 1764–1769 (2013).
Snaith, H. J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 4, 3623–3630 (2013).
Jeng, J.-Y. et al. CH3NH3PbI3 Perovskite/Fullerene Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 25, 3727–3732 (2013).
Docampo, P., Ball, J. M., Darwich, M., Eperon, G. E. & Snaith, H. J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat Commun 4, 2761 (2013).
Sun, S. et al. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 7, 399–407 (2014).
Ku, Z., Rong, Y., Xu, M., Liu, T. & Han, H. Full Printable Processed Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells with Carbon Counter Electrode. Sci. Rep. 3, 3132 (2013).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Liu, M., Johnston, M. B. & Snaith, H. J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501, 395–398 (2013).
Liu, D. & Kelly, T. L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nature Photon. 8, 133–138 (2014).
Park, N.-G. Organometal Perovskite Light Absorbers Toward a 20% Efficiency Low-Cost Solid-State Mesoscopic Solar Cell. J. Phys. Chem. Lett. 4, 2423–2427 (2013).
Stranks, S. D. et al. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 342, 341–344 (2013).
Xing, G. et al. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 342, 344–347 (2013).
Gonzalez-Pedro, V. et al. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 14, 888–893 (2014).
Heo, J. H. et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nature Photon. 7, 487–492 (2013).
Kumar, M. H. et al. Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Comm. 49, 11089–11091 (2013).
Eperon, G. E., Burlakov, V. M., Docampo, P., Goriely, A. & Snaith, H. J. Morphological Control for High Performance, Solution- Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 24, 151–157 (2014).
Chen, Q. et al. Planar Heterojunction Perovskite Solar Cells via Vapor-Assisted Solution Process. J. Am. Chem. Soc. (2013).
Malinkiewicz, O. et al. Perovskite solar cells employing organic charge-transport layers. Nature Photon. 8, 128–132 (2014).
Qiu, J. et al. All-solid-state hybrid solar cells based on a new organometal halide perovskite sensitizer and one-dimensional TiO2 nanowire arrays. Nanoscale 5, 3245–3248 (2013).
Kim, H.-S. et al. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 Nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 13, 2412–2417 (2013).
Etgar, L. et al. Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells. J. Am. Chem. Soc. 134, 17396–17399 (2012).
Laban, W. A. & Etgar, L. Depleted hole conductor-free lead halide iodide heterojunction solar cells. Energy Environ. Sci. 6, 3249–3253 (2013).
Bi, D. et al. Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells. Nanoscale 5, 11686–11691 (2013).
Conings, B. et al. Perovskite-Based Hybrid Solar Cells Exceeding 10% Efficiency with High Reproducibility Using a Thin Film Sandwich Approach. Adv. Mater., 10.1002/adma.201304803 (2013).
Cai, B., Xing, Y., Yang, Z., Zhang, W.-H. & Qiu, J. High performance hybrid solar cells sensitized by organolead halide perovskites. Energy Environ. Sci. 6, 1480–1485 (2013).
Abrusci, A. et al. High-Performance Perovskite-Polymer Hybrid Solar Cells via Electronic Coupling with Fullerene Monolayers. Nano Lett. 13, 3124–3128 (2013).
Noh, J. H. et al. Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/Co-complex as hole-transporting material. J. Mater. Chem. A 1, 11842–11847 (2013).
Jeon, N. J. et al. Efficient Inorganic–Organic Hybrid Perovskite Solar Cells Based on Pyrene Arylamine Derivatives as Hole-Transporting Materials. J. Am. Chem. Soc. 135, 19087–19090 (2013).
Di Giacomo, F. et al. High efficiency CH3NH3PbI(3−x)Clx perovskite solar cells with poly(3-hexylthiophene) hole transport layer. J. Power Sources 251, 152–156 (2014).
Christians, J. A., Fung, R. C. M. & Kamat, P. V. An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide. J. Am. Chem. Soc. 136, 758–764 (2014).
Irwin, M. D., Buchholz, D. B., Hains, A. W., Chang, R. P. H. & Marks, T. J. p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. PNAS 105, 2783–2787 (2008).
Nattestad, A. et al. Highly efficient photocathodes for dye-sensitized tandem solar cells. Nature Mater. 9, 31–35 (2010).
Powar, S. et al. Highly Efficient p-Type Dye-Sensitized Solar Cells based on Tris(1,2-diaminoethane)Cobalt(II)/(III) Electrolytes. Angew. Chem. Int. Ed. 52, 602–605 (2013).
Gibson, E. A. et al. A p-Type NiO-Based Dye-Sensitized Solar Cell with an Open-Circuit Voltage of 0.35 V. Angew. Chem. Int. Ed. 48, 4402–4405 (2009).
Ratcliff, E. L. et al. Investigating the Influence of Interfacial Contact Properties on Open Circuit Voltages in Organic Photovoltaic Performance: Work Function Versus Selectivity. Adv. Energy Mater. 3, 647–656 (2013).
Odobel, F. & Pellegrin, Y. Recent Advances in the Sensitization of Wide-Band-Gap Nanostructured p-Type Semiconductors. Photovoltaic and Photocatalytic Applications. J. Phys. Chem. Lett. 4, 2551–2564 (2013).
Garcia, A. et al. Improvement of Interfacial Contacts for New Small- Molecule Bulk-Heterojunction Organic Photovoltaics. Adv. Mater. 24, 5368–5373 (2012).
Manders, J. R. et al. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 23, 2993–3001 (2013).
Jeng, J.-Y. et al. Nickel Oxide Electrode Interlayer in CH3NH3PbI3 Perovskite/PCBM Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 10.1002/adma.201306217 (2014).
Mühlbacher, D. et al. High Photovoltaic Performance of a Low-Bandgap Polymer. Adv. Mater. 18, 2884–2889 (2006).
Wienk, M. M., Turbiez, M., Gilot, J. & Janssen, R. A. J. Narrow-Bandgap Diketo-Pyrrolo-Pyrrole Polymer Solar Cells: The Effect of Processing on the Performance. Adv. Mater. 20, 2556–2560 (2008).
Liang, Y. et al. Development of New Semiconducting Polymers for High Performance Solar Cells. J. Am. Chem. Soc. 131, 56–57 (2008).
Huang, F. et al. Development of New Conjugated Polymers with Donor−π-Bridge−Acceptor Side Chains for High Performance Solar Cells. J. Am. Chem. Soc. 131, 13886–13887 (2009).
Boschloo, G. & Hagfeldt, A. Spectroelectrochemistry of Nanostructured NiO. J. Phys. Chem. B 105, 3039–3044 (2001).
You, J. et al. Low-Temperature Solution-Processed Perovskite Solar Cells with High Efficiency and Flexibility. Acs Nano 8, 1674–1680 (2014).
Chiang, Y.-F. et al. High voltage and efficient bilayer heterojunction solar cells based on organic-inorganic hybrid perovskite absorber with low-cost flexible substrate. Phys. Chem. Chem. Phys. 16, 6033–6040 (2014).
Borgström, M. et al. Sensitized Hole Injection of Phosphorus Porphyrin into NiO: Toward New Photovoltaic Devices. J. Phys. Chem. B 109, 22928–22934 (2005).
Acknowledgements
P.C. appreciates the research funding from National Science Council (NSC) of Taiwan (NSC102-2113-M-006-010). T.F.G. would like to thank the NSC of Taiwan (NSC102-2682-M-006-001-MY3) for financially supporting this research. E.W.G.D. acknowledges the support from NSC of Taiwan (NSC102-2113-M- 009-020-MY3).
Author information
Authors and Affiliations
Contributions
P.C. and T.-F.G. both proposed the research and supervised the whole project. K.-C.W., J.-Y.J. and P.-S.S. carried out the experiments for device fabrication, optimization and characterizations. P.C., T.-F.G. and E.W.-G.D. contributed to the data analysis and interpretation for the results. K.-C.W. performed the HR-SEM measurements. C.-H.T., T.-Y.C. and H.-C.H. measured the PL spectra. P.-Y.L. prepare the NiOx and perovskite precursor materials. E.W.-G.D. supervised the PIA measurements while Y.-C.C. conducted the experiments. T.-C.W. supervised the preparation of materials and participated the discussion. P.C. wrote the manuscript with further inputs from all the other authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wang, KC., Jeng, JY., Shen, PS. et al. p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells. Sci Rep 4, 4756 (2014). https://doi.org/10.1038/srep04756
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04756
This article is cited by
-
Numerical analysis of all-inorganic perovskite solar cells with different Cu-based hole transport layers under indoor illuminations
Optical and Quantum Electronics (2024)
-
Aerosol-assisted CVD of nickel oxide on silicon for hole selective contact layers
Journal of Materials Science: Materials in Electronics (2023)
-
Advances in the Application of Perovskite Materials
Nano-Micro Letters (2023)
-
The effect of mechanically milled lead iodide powder on perovskite film morphology
Applied Physics A (2022)
-
Efficiency improvement of perovskite solar cell by modifying structural parameters and using Ag nanoparticles
Applied Physics A (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.