Abstract
A life-history in which an organism depends on ants is called myrmecophily. Among Lepidoptera (moths and butterflies), many species of lycaenid butterflies are known to show myrmecophily at the larval stage. Descriptions of myrmecophily among moth species, however, are very few and fragmentary. Here, we report the ant-associated behaviour of the tiny Japanese arctiid moth, Nudina artaxidia. Field observations revealed that the moth larvae associate with the jet black ant, Lasius (Dendrolasius) spp. The larvae, which we observed only near ant trails, showed an ability to follow the trails. Further, they solicit honeydew from ant-attended scale insects, without suffering attacks by the ants protecting the scale insects. These suggest that N. artaxidia is a myrmecophilous moth wholly dependent on ants and ant-attended homopterans. Considering the overwhelmingly plant-feeding habits of moth caterpillars, this discovery ranks in novelty with the discovery of the Hawaiian carnivorous moth larvae that stalk snails.
Similar content being viewed by others
Introduction
A life-history strategy in which an organism depends wholly or partially on ants is called myrmecophily1. In invertebrates, the vast majority of obligate myrmecophiles are arthropods2,3. Lepidoptera (moths and butterflies) are among the most diverse insect groups, but they have overwhelmingly constrained feeding habits (plant-feeders). Of c.150,000 species of moths and butterflies, less than 0.2% are specialized predators (mostly belonging to Lycaenidae butterflies) and still few, several anomalous butterfly species feed on homopteran honeydew4,5. The association of lycaenid butterflies with ants can be mutualistic or parasitic: plant-feeding lycaenid mutualists are protected by ants and reward the ants by giving them nutritious secretions, whilst predatory or parasitic lycaenids feed on ant-attended homopterans, the ants themselves, or ant regurgitations6,7,8. In contrast to the numerous studies on lycaenid myrmecophily, reports on myrmecophily in moths are scarce and fragmentary. For example, although Pierce4 lists many predatory and parasitic lepidopteran taxa, including some 130 moth species, she focuses on the myrmecophilous habits of lycaenid butterflies. According to Pierce4, most predatory moths consume homopterans (especially sessile scale insects) but nine species consume ants. Therefore, some association of predacious moth larvae with ants is possible.
Moths of family Arctiidae are overwhelmingly phytophagous and feed on various plants and lichens9, but some species are carnivorous9,10,11. A myrmecophilous-like habit has been reported only in one Northern American species of Arctiidae, Crambidia casta (Packard), whose larvae stay near Formica ant nests and feeds on lichens around the nests12. Here, we report the ant-associated behaviours of a myrmecophilous moth in Japan. The larvae of the arctiid moth Nudina artaxidia (Butler) (Lithosiinae) are found only along and within jet black ant Lasius (subgenus Dendrolasius) trails and they feed on honeydew produced by ant-attended homopterans as well as on lichens growing along the trails.
Results
Behaviour around ant nests
We found four nests of Lasius (Dendrolasius) nipponensis, two of L. (D.) capitatus and four of L. (D.) fuji. Among the surveyed Dendrolasius nests, we found N. artaxidia larvae at four L. nipponensis nests, one L. capitatus nest and one L. fuji nest. At each of these nests, we found from zero to three N. artaxidia larvae each night, most frequently in April. The N. artaxidia larvae that we found could all follow ant trails without suffering ant attacks (Fig. 1) and we found no larvae more than a meter from either an ant trail or the entrance to an ant nest.
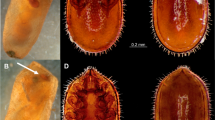
Larvae of Nudina artaxidia on a Zelkova serrata trunk.
(a), Following a Lasius (Dendrolasius) capitatus trail; (b), stealing honeydew from a giant scale insect, Drosicha corpulenta, being attended by L. (D.) nipponensis. Each scale bar represents 5 mm. This photograph was originally taken by Takashi Komatsu, corresponding author.
Feeding habit
When a N. artaxidia larvae approached an ant clump to steal honeydew, the ants were attending and collecting honeydew from individuals of the giant scale insect, Drosicha corpulenta. The N. artaxidia larva attempting to steal honeydew slowly approached the ants gathered around a scale insect while shaking its head. After it reached the side of the scale insect, it tenaciously beat the insect's anus with its head. As soon as the scale insect secreted a honeydew droplet, the larva sucked it up (Fig. 1). The mean numbers of the beating behaviour per minute were 9.5 for Individual A and 9.8 for Individual B, respectively and were not significantly different between the individuals (Mann-Whitney's U test, P = 0.936) (Fig. 2). Although most of the surrounding ants did not react in a hostile manner toward the feeding larva, a few ants showed threat behaviour (but not attack behaviour). This honeydew-feeding behaviour was performed for about 10 minutes. On two other occasions, on 24 July 2010 at Jyo-yama and on 12 September 2011 at Satoyamabe, we found a larva feeding on lichens growing on tree bark by the side of an L. nipponensis trail. In these instances, the larva temporarily left the ant trail to feed on lichens growing within a few centimetres of the ant trail and after feeding, each larva returned to the ant trail.
The number of times of beating behaviour (soliciting behaviour for honeydew from scale insects) recognized in larvae of Nudina artaxidia (Individuals A and B).
The box plot represents 25th, 50th and 75th percentiles. The top and bottom whiskers represent largest and smallest nonoutlier observations, respectively.
Discussion
Most species of Lithosiinae are regarded as lichen-feeding moths13,14, so it was not surprising to find N. artaxidia feeding on lichens (hitherto, the food preferences of this species have been unknown14). Similarly, larvae of the American arctiid, C. casta12, also feed on lichens and stay near ant nests without being attacked by the ants. However, N. artaxidia is quite remarkable because it actively feeds on honeydew produced by homopterans being protected by ants and because its larvae are found only within Dendrolasius ant territories. The host Dendrolasius ants have large mandibular glands that produce specific chemicals, such as dendrolasin and citronellal3 that are strongly toxic to other insects15, so N. artaxidia may have evolved a tolerance to such ant-derived toxic chemicals. Both N. artaxidia and Dendrolasius ants occur in Siberia, Korea, China, Japan and Taiwan13,14,16 and the congruence of their distributions suggests that N. artaxidia may be intimately associated with Dendrolasius ants over its whole distribution range.
It may be advantageous for N. artaxidia larvae to live along the trails and near the nests of poisonous ants because it keeps them free of parasitic enemies and allows them to feed without being vulnerable to attacks by predators. Moreover, in preliminary laboratory experiments, three early-instar N. artaxidia larvae fed only on lichens died before pupation (T. K., personal observation), a result that suggests that homopteran honeydew may be nutritionally necessary for normal larval development. As homopteran honeydew contains considerable amount of amino acid17,18, it leastwise offers important nitrogen source for N. artaxidia. On the other hands, lichen itself that is alternative food of N. artaxidia is also nitrogen-rich food resource19. Further rearing experiments without homopteran honeydew would reveal how much larval N. artaxidia depends on honeydew as essential food resource.
On the other hand, N. artaxidia larvae were not observed to interact directly with ants, as some myrmecophilous arthropods do, for example, by mouth-to-mouth feeding20,21,22. As with the larvae of lipteninae Lycaenidae23,24,25, long hairs cover the entire body of N. artaxidia larvae (Fig. 1), which may preclude physical contact with the ants. Similar to the lycaenid butterfly Shirozua jonasi26, N. artaxidia larvae appear to depend on ants only for defence from their enemies and not for nutrition from ants directly via mouth-to-mouth feeding. A similar lack of direct contact with ants is also observed in the American arctiid C. casta12. In both arctiid species, it is uncertain whether the lack of concern shown by ants is due to chemical mimicry on the part of the larvae.
The phylogenetic relationship between the Eurasian genus Nudina and the American genus Crambidia (both of which belong to the lichen moth subfamily Lithosiinae) is unknown, because a comprehensive phylogenetic analysis of Arctiidae has yet to be performed. Further, a relationship between members of these moth genera and ants has only been reported for N. artaxidia (this paper) and C. casta12. Therefore, any evaluation of the origins of myrmecophilic behaviour in Arctiidae would require more extensive phylogenetic and life history analyses. The case of N. artaxidia is notable, compared with C. casta, because N. artaxidia displays more integrated traits, such as resistance to ant-derived toxins and specific soliciting behaviour to acquire homopteran honeydew. Thus, N. artaxidia is the only myrmecophilous arctiid so far recognized to be wholly dependent on host ants and it is the first moth species that has been documented to solicit honeydew from ant-protected homopterans. Considering the constrained feeding habits of moth caterpillars, evolution to solicit and feed on homopteran honeydew is an anomaly. N. artaxidia and related taxa such as C. casta are ideal model systems in which to examine the unexpected evolution of myrmecophily in Lepidoptera and for comparisons of myrmecophilic moths with the better known myrmecophilic Lycaenid butterflies.
Methods
Behaviour around ant nests
Several ant species of the subgenus Dendrolasius live in secondary oak-pine forests in Matsumoto, Nagano, Japan27, where the dominant tree species are Quercus acutissima and Pinus densiflora. We carried out a field survey in Matsumoto from 2010 to 2013 to find nests of Dendrolasius. Because preliminary observations had suggested that larval N. artaxidia could be found on the ground or on tree trunks near ant trails after sunset, we searched the ground and tree trunks around ant nests every night (19:00–21:00 LT) from March to October to find N. artaxidia larvae.
Feeding habit
During above-mentioned survey, we observed honeydew-feeding behaviour of two N. artaxidia larvae near L. nipponensis trails, one on a Quercus sp. trunk at Jyo-yama on 31 May 2010 (Individual A) and the other on a Zelkova serrata trunk at Satoyamabe on 29 May 2013 (Individual B). We defined behaviour of the caterpillars that beat the scale insects at the anus by oscillating movements of their mouthparts as ‘soliciting behaviour’ for honeydew. We recorded the soliciting behaviour of the caterpillars by digital movie camera (1 minute for both Individual A and B) and counted the number of times of beating behaviour per minute. The number between the two individuals was compared by Mann-Whitney's U test.
References
Maruyama, M. et al. The guests of Japanese ants (Tokai University Press, 2013).
Kistner, D. H. [The social insect' bestiary] Social Insects vol. III [Hermann, H. R. (ed.)] [1–244] (Academic Press, 1982).
Hölldobler, B. & Wilson, E. O. The Ants (Belknap Press of Harvard University, 1990).
Pierce, N. E. Predatory and parasitic Lepidoptera: carnivores living on plants. J. Lepidopterists' Soc. 49, 412–453 (1995).
Fiedler, K. The remarkable biology of two Malaysian lycaenid butterflies. Nature Malaysiana 18, 35–43 (1993).
Hinton, H. E. Myrmecophilous Lycaenidae and other Lepidoptera -- a summary. Trans. So. Lond. Entomol. Nat. Hist. Soc. 1949–1950, 111–175 (1951).
Wilson, E. O. The Insect Societies (Belknap Press, 1971).
Pierce, N. E. et al. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 47, 733–771 (2002).
Wagner, D. L. [The immature stages: structure, function, behaviour and ecology] Tiger Moths and Woolly Bears: Behavior, Ecology and Evolution of the Arctiidae [Conner, W. E. (ed.)] [31–54] (Oxford University Press, 2008).
Krasnoff, S. B. & Roelofs, W. L. Quantitative and qualitative effects of larval diet on male scent secretions of Estigmene acrea, Phragmatobia foliginosa and Pyrrharctia isabella (Lepidoptera: Arctiidae). J. Chem. Ecol. 15, 1077–1093 (1989).
Wagner, D. L. Caterpillars of Eastern North America: a Guide to Identification and Natural History (Princeton University Press, 2005).
Ayre, G. Notes on insects found in or near nests of Formica subnitens Creighton (Hymenoptera: Formicidae) in British Columbia. Insec. Soc. 5, 1–7 (1958).
Inoue, H. [Arctiidae] Moths of Japan volume 1: Text [Inoue, H. et al. (eds.)], [638–659] (Kodansha, 1982).
Kishida, Y. [Arctiidae] The Standard of Moths in Japan II [Kishida, Y. et al. (eds.)] [148–167] (Gakken, 2011).
Buschinger, A. & Maschwitz, U. [Defensive behavior and defensive mechanisms in ants] Defensive Mechanisms in Social Insects [Hermann, H. R.] (ed) [95–150] (Praeger, 1984).
Myrmecological Society of Japan. A Guide for the Identification of Japanese Ants II. Dolichoderinae and Formicinae (Hymenoptera: Formicidae) (The Myrmecol. Soc. Japan, 1991).
Maurizio, A. [Honigtau-honigtauhonig] Wladtracht und Waldhonig in der Imkerei [Kloft, W. J. et al. (eds.)] [268–295] (Ehrenwirth, 1985)
Auclair, J. L. Aphid feeding and nutrition. Annu. Rev. Entontol. 8, 439–490 (1963).
Balduf, W. V. The rise of entomophagy among Lepidoptera. Am. Nat 72, 358–379 (1938).
Wheeler, W. M. Studies on myrmecophiles, II: Hetaerius. J. N. Y. Entomol. Soc. 16, 135–143 (1908).
Elmes, G. W. et al. Larvae of Maculinea rebeli, a large-blue butterfly and their Myrmica host ants: wild adoption and behaviour in ant-nests. J. Zool. 223, 447–460 (1991).
Ito, F. & Takaku, G. Obligate myrmecophily in an oribatid mite: novel symbiont of ants in the Oriental tropics. Naturwissenschaften 81, 180–182 (1994).
Clark, G. C. & Dickson, C. G. C. Life Histories of Southern African Lycaenid Butterflies (Purnell, 1971).
Rosier, J. P. Notes on Lepidoptera. II. Metamorphosis of some Javanese butterflies. Idea 9, 26 (1951).
Eliot, J. N. The higher classification of the Lycaenidae (Lepidoptera): a tentative arrangement. Bull. Br. Mus. Nat. Hist. 28, 373–505 (1973).
Yamaguchi, S. The Life Histories of Five Myrmecophilous Lycaenid Butterflies of Japan (Kodansha, 1988).
Komatsu, T. & Konishi, K. Parasitic behaviors of two ant parasitoid wasps (Ichneumonidae: Hybrizontinae). Sociobiology 56, 575–584 (2010).
Acknowledgements
We thank U. Jinbo for information about the natural history of Lithosiinae moths and S. Duhon for English editing.
Author information
Authors and Affiliations
Contributions
T.K. designed this study with input from T.I., performed the research and analysed the data. T.K. and T.I. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Komatsu, T., Itino, T. Moth caterpillar solicits for homopteran honeydew. Sci Rep 4, 3922 (2014). https://doi.org/10.1038/srep03922
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03922
This article is cited by
-
A cuckoo-like parasitic moth leads African weaver ant colonies to their ruin
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.