Abstract
Tailoring the nano-morphology and nano-architecture of titanium dioxide (TiO2) is the most important task in the third generation solar cells (Dye sensitized solar cells/Quantum dot sensitized solar cells) (DSSCs/QDSSCs). In this article we present complete study of surfactant free synthesis of TiO2 nanostructures by a simple and promising hydrothermal route. The plethora of nanostructures like nanoparticles clusters, 1D tetragonal nanorods, 3D dendrites containing nanorods having <30 nm diameter and 3D hollow urchin like have been synthesized. These nanostructures possess effective large surface area and thus useful in DSSCs. In the present work, 7.16% power conversion efficiency has been demonstrated for 3D dendritic hollow urchin like morphology. Our synthetic strategy provides an effective solution for surfactant free synthesis of efficient TiO2 nanoarchitectures.
Similar content being viewed by others
Introduction
After the discovery of photoelectrochemical properties of nanostructured titanium oxide (TiO2), it is recognized as one of most promising wide band gap semiconducting materials for photocatalysis, dye/quantum dot sensitized solar cells (DSSCs/QDSSCs) and lithium ion batteries1,2,3,4,5,6. The DSSC is a molecular approach to photovoltaic solar energy conversion technology. This is one of the emerging photovoltaic technologies that offer the potential to reduce the cost of photovoltaic electricity production. During the past two decades, nanoporous polycrystalline titania has been extensively used in DSSCs, which demonstrated to be a promising alternative to silicon based solar cells due to their relatively high solar-to-electric power conversion efficiency at low cost. The transportation of electrons through TiO2 film and effective dye loading are the most important parameters in DSSCs. These two parameters depend upon the surface topography of the photoanode, surface area, grain boundaries between two nanostructures and porosity of the photoanodes. Hence, the tailoring nanomorphology of photoanode is a key factor in the DSSCs application2.
TiO2 mainly occurs in three main crystal phases: anatase, rutile and brookite. However, synthesis of one/three dimensional (1D/3D) growth of any one of these nanostructures is a difficult task. Recently many attempts have been made to tackle this problem using strong acid reaction7, ionic liquid surfactant mediator8, dissolve and grow process9. Recently, D.-B. Kuang et al. have developed a new oriented hierarchical single crystalline anatase TiO2 nanowire arrays, tri-functional spheres consisting nanorods and hierarchical nanowire trunks by hydrothermal process10,11,12. C. Lin et al. reported porous rutile TiO2 nanorod arrays etching process13. However till now there is no substantial work on surfactant free hydrothermal process for synthesis of nanostructured TiO2 using Titanium butoxide (Ti(OC4H9)4) (TBT) precursor. On the other hand 1D nanostructures provide slow recombination rate, fast electron transport and effective light scattering ability within the nanostructures. The 3D nanostructure like nanoflowers14, hierarchical microspheres15,16 functioning high specific surface area results in an effective dye adsorptive and light-scattering layer. To achieve this we have developed surfactant free hydrothermal synthesis route for 1D as well as 3D TiO2 nanostructures with well-defined shape and size. Such novel 1D nanorods arrays with 3D dendrites and hollow urchin provide not only effective surface area but are also helpful for effective light harvesting in DSSCs. The present study is focused on the effect of temperature on hydrolysis of TBT precursor for tuning of TiO2 nanomorphology and its DSSCs performance discussed systematically. The key innovation in the present study is to demonstrate surfactant free tuning of the nanomorphology of TiO2 nanostructures by a controlled single step hydrothermal process at various system temperatures. The TBT was controlled hydrolyzed in hydrochloric acid and distilled water (1:1 v:v). The reaction temperature was varied from 100°C to 190°C and growth mechanism is studied systematically. Finally these nanostructures were used for DSSCs application.
Results
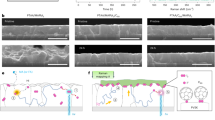
Figure 1 shows typical FESEM images of TiO2 nanostructures synthesized at different reaction temperatures. The reaction time was 3 h for each sample deposition. Figure 1 (a) show the FESEM images of TiO2 synthesized at 100°C (T100) on the FTO coated conducting substrate. The compact TiO2 nanoparticles clusters are deposited on entire surface of the FTO substrate. The particle sizes of the deposited nanoparticles were found to be 25–35 nm. Figure 1 (b) show FESEM images of TiO2 sample at 110°C (T110), revealing tapered nanorods having 180 nm diameter. However, the highly magnified image shows that these large size nanorods are made up from agglomeration of number of small nanorods (pillars). Therefore we have decided to increase the hydrothermal system temperature. Figure 1 (c) show FESEM images of TiO2 nanorods deposited at 120°C designated as T120. Uniform distribution of vertically aligned nanorods covered throughout the substrate. Figure 1 (d) show the sample morphology deposited at 130°C (T130). The previously agglomerated nanorods start separating into much smaller (25–35 nm) nanorods. Figure 1 (e) shows FESEM image of T140 sample. There are no drastic changes observed for T140 sample except small inter-nanostructure spacing. However T150 sample shows excellent inter-nanostructure separation between two bunches of nanorods (Figure 1 (f)). These nanorods are covered uniformly over entire surface having tetragonal shape with square top facets. Notably these tetragonal nanorods are vertically aligned to the FTO substrate (Please check electronic Supporting Information Figure S1 and Figure S2). The cross sectional FESEM image shows TiO2 nanorods were uniformly distributed and aligned vertically to the FTO substrate. The thickness of deposited sample is ~6.6 μm throughout surface. The inset shows highly magnified FESEM image of selected area that reveals bunch of aligned nanorods. The TiO2 nanorod and FTO interface is very smooth, which is beneficial for effective flow of electrons.
Exotic Nanostructures of hydrothermally grown 1D/3D TiO2 at different hydrolysis conditions.
(a) T100, (b) T110, (c) T120, (d) T130, (e) T140, (f) T150, (g) T160, (h) T170, (i) T180 and (j) T190. The images on right hand show their respective highly magnified FESEM micrographs. (k) Photograph of a hollow platanus seed found in the nature that mimics the nanostructures of T190 sample. (Image Credit: Dr. Sawanta S. Mali).
It is well known that the nanorods are tetragonal in shape with square top facets, the expected growth habit for the tetragonal crystal structure. Similar morphology has been observed by E. Hosono et al. and Z. L. Wang et al.17,9 by hydrolysis of the TiCl3 in NaCl solvent at 200°C and Ti foil surface etching process in strong acidic concentrated HCl solution on carbon nanofiber for ~18 h respectively. Moreover, S. A. Berhe et al.18 also reported similar morphology by hydrolysis of titanium alkoxide in HCl solution by two consecutive 6 h growths on seed coated MoO2 substrate. Here we could obtain same bundles of enclosing a few or several nanorods in relatively less time, using surfactant free solution and seed free substrate. The sample deposited at 160°C (i.e. Sample-T160) shows novel nanoflower like morphology having bunch of aligned nanorods. The diameter of such flower is about 3 μm as shown Figure 1 (g). However, T170 sample shows well distributed TiO2 nanoflowers over the substrate as shown in Figure 1 (h). The image on right hand side shows clearly these nanorods containing bunch of aligned nanorods. The cross section FESEM images shows the dendrites are tapered and centered at the core of the nanoflower (Please check supporting information Figure S3). These nanorods are single crystalline in nature confirmed by spotted SAED pattern19,20,21.
E. Hosono et al.17 and recently Z. L. Wang et al.9 discussed the recrystallization process i.e. dissolve and grow process of TiO2 nanostructures by hydrothermal process. The hydrolysis reaction in strong acidic media can be explained as follows:



The XRD pattern of the TiO2 sample deposited onto FTO substrate is shown in Figure S4 (Supporting Information Figure S4). The reflection peaks can be readily indexed to pure rutile TiO2. The other peaks are originated from FTO substrate. The proposed formation of 1D nanorods growth is as follows:
Initially Ti species from TBT precursor start to react with H+ ions from concentrated solution. It is well known that Ti3+ species are not stable in an aqueous solution, therefore TiOH2+ species are formed by hydrolysis of Ti3+ species. According to the “dissolve and grow method9,15,17 TiOH2+ is oxidized to Ti(IV) by reaction with dissolved oxygen. The Ti(IV) complex ions are thus used as the growth units. The formation mechanism of the rutile TiO2 NRs may be described as follows: For rutile TiO2, a TiO6 octahedron forms first by bonding of a Ti atom and six oxygen atoms. The TiO6 octahedron then shares a pair of opposite edges with the next octahedron, forming a chainlike structure. Because the growth rate of the different crystal faces depends on the numbers of corners and edges of the coordination polyhedra available, the growth of rutile NRs follows the sequence (110) < (100) < (101) < (001)17,22,23,24. Thus, rutile TiO2 NRs along [001] direction are formed. The nanorods are single crystalline, as evidenced by the sharp spotted SAED pattern of a nanorod examined along the [110] zone axis. The chemical stoichiometry of the nanorods was further examined with X-ray photoelectron spectroscopy (XPS) and the atomic ratio of Ti to O was found to be ~1:2 (Please check electronic Supporting Information, Figure S5).
Figure 1 (i) show the FESEM images of T180 sample. The deposited samples exhibit 3D dendritic TiO2 nanostructures containing nanorods. These dendritic microspheres are ~7.5 μm in diameter. Interestingly the highly magnified image shows uniform distribution of tetragonal nanorods of ~25–35 nm diameter. Similar morphology has been reported by J. H. Kim et al.25,26 with a composition of 100H2O:7HCl:0.03CTAB:0.05 0.01TTIP (where CTAB: Cetyl trimethylammonium bromide, TTIP: Titanium tetraixopropoxide) as a solution composition for 20 h hydrothermal process. In our case, after increasing the hydrothermal system temperature it is observed that the 3D dendritic nanostructured microspheres open at the outer surface (Figure 1 (j)). Such novel hollow urchin nanostructures have 1 μm diameter. This 3D dendritic hollow urchin structure may have formed due to selected surface etching of Ti species in strong acid medium at relatively higher temperature7. A magnified FESEM image of the sample in Figure 1 (j) shows that these 3D dendrites mimics the hollow platanus seed (Figure 1 (k)), with diameters of 1 μm and numerous nanorods (~30 nm) compactly growing around their surfaces.
The crystallinity of deposited samples was confirmed by SAED, TEM and HRTEM characterizations. Figure 2 show the TEM, SAED patterns and HRTEM images of selected TiO2 samples: (a–e) Sample deposited at 140°C (T140). (f–j) sample deposited at 160°C (T160) and (k–o) sample deposited at 190°C (T190). The T140 sample exhibits the nanorods with tapered morphology (Fig. 2(a)). The agglomerated tetragonal nanorods are separated at the upper side (head) while lower side (tail) is compact in nature. The highly magnified image of the tail (Figure 2 (b)) reveals bundles of tiny nanorods. The diameter of the tail side is ~80 nm.
Transmission Electron Microscopic (TEM) images, selective area diffraction (SAED) patterns and high resolution transmission electron microscopic images (HRTEM) of selected TiO2 samples: (a–e) Sample deposited at 140°C (T140). (f–j) sample deposited at 160°C (T160) and (k–o) sample deposited at 190°C (T190).
Figure 2 (c) shows highly magnified TEM image of upper side (head) of the tetragonal nanorods. It is clear that the nanorods are separated at the head side with ~30 nm diameter of each nanorod. Moreover, these nanorods are single crystalline as confirmed by their SAED pattern (Figure 2 (d)). The clear lattice fringes of the single nanorod of the T140 sample is observed to be single crystalline along their entire length. The interplanar spacing obtained from the HRTEM lattice fringes along d110 = 0.32 nm between the adjacent lattice fringes perpendicular to the rod axis can be assigned to the rutile TiO2 (110). The lattice spacing of d001 = 0.29 nm along the longitudinal axis direction pertains to the d-spacing of rutile TiO2 (001) crystal planes14. Figure 2 (f–j) presents the TEM images of T160 sample. These images are almost similar to sample T140 however the separation of the upper side is slight higher. Also similar lattice spacings along [110] and [001] directions have been observed. Figure 2 (k–o) shows the TEM images of the T190 sample. The SAED pattern shows single crystalline nature of the 3D hollow urchin like morphology of the sample (T190). The growth direction of the tetragonal nanorods in the 3D TiO2 dendrites were in [001] direction, which is the same as for the 1D vertically grown nanorods. The exposed surfaces of the nanorods were {110} facets25. Interestingly it is observed that the crystallinity of the T190 sample is better than other samples.
From the above discussion the possible growth mechanism of nanoparticles-to-nanorods-to-3D dendrite microspheres-to-3D hollow urchin-like architectures is represented in Figure 3. Here we have used strong acid approach for the synthesis of 1D and 3D nanostructures. It is well known that the equal volume of HCl:H2O is beneficial for the synthesis of aligned TiO2 nanorods7,21. In our procedure we have kept HCl:H2O (1:1 v:v) fixed throughout the experiments. The concentrated HCl constraint on the hydrolysis of the TiO2 precursor results in 1D TiO2 nanorods. The growth of oriented TiO2 nanorods requires slow hydrolysis of TBT in a fairly strong acidic aqueous medium. However the system temperature also plays a key role in the complete hydrolysis of titanium precursor. At 100°C (T100) insufficient system temperature causes creation of clusters of nanoparticles. When the FTO substrates were immersed in the reaction solutions, titanium precursor would condense on the FTO surface and growth of TiO2 seeds starts26. At 130°C (T100 sample), pillars of aligned nanorods are formed due to controlled hydrolysis. The increase rate of hydrolysis facilitates rapid formation of nanorods that causes 3D growth of bunch of nanorods, at 160°C (T160) and 170°C (T170). However, drastic modification has been observed for T180 sample. The 3D spherical dendrites of diameter ~7.5 μm containing nanorods of size ~30 nm have been formed. Similar microstructures have been synthesized by J. H. Kim et al.25. using 100H2O:7HCl:0.03CTAB:0.05TTIP. Here authors have used CTAB as a surfactant and concluded that the surfactant is helpful for the aligned growth of TiO2 nanorods. However it is also concluded that such common surfactants are not playing major role in the tuning and reproducibility of nanomorphology7.
At 190°C, the sufficiently high temperature causes formation of 3D hollow urchin like morphology due to higher surface energy. The low magnified FESEM image shows uniformity of the hollow urchin structure. (Supporting information Figure S6).
Further the statistical distribution of nanorod diameter of 3D dendritic sample is estimated. Figure 4 shows a statistical histogram of the diameter distribution of the hydrothermally grown 3D TiO2 dendrites. The inset shows the selected area for this measurement. It is clear that the large numbers of 1D nanorods are in the range of 25–30 nm.
The DSSC based on 3D nanostructures like nanoflowers8, hierarchical microspheres9,10 result in high surface area and susequently enable effective dye adsorptiion. Recently Z. Sun et al. demonstrated nanowire/dendritic 3D nanostructures useful for effectively light harvesting in DSSCs. These 1D-3D nanostructures show 7.2% photon conversion efficiency (PCE). Therefore, 3D nanostructures combined with 1D nanostructures has opened a new approach towards efficient DSSCs27. Therefore we have decided to use such 1D/3D nanostructure in well-known DSSC application. The DSSC devices in this study were fabricated by following standard procedure10. The N-719 dye was used for sensitization and Pt/FTO was used as a counter electrode. The samples show good solar cell properties under 100 mW/cm2 AM 1.5 illumination and the results are summarized in Table 1.
Figure 5 shows the J-V curves of TiO2 sample based on different morphologies. For nanoparticle T100 sample based DSSC cell short-circuit current density (JSC), open-circuit voltage (VOC), fill factor (FF) and efficiency (η) were JSC = 7.85 mAcm−2, Voc = 0.587 V, FF = 48.2 and η = 2.34%, respectively. DSSC device based of T110 sample shows JSC = 9.42 mAcm−2, VOC = 0.589 V, FF = 54 and η = 2.99%. Sample T120 shows 3.86% conversion efficiency with 9.53 mAcm−2 Jsc and 0.599 VOC respectively. Interestingly it was found that the current density (9.79 mAcm−2) as well as efficiency (4.11%) of the T130 sample is larger than nanoparticulate clusters and compact nanorods pillars. This may be due to higher surface area beneficial for effective dye loading. The T140 sample shows slightly higher 4.14% conversion efficiency. The T150 sample shows VOC = 0.610 V, JSC = 12.59 mAcm−2, FF = 61.1 and η = 4.93%. While the 3D TiO2 nanoflower sample shows drastic enhancement in power conversion efficiency. These 3D nanoflowers (Sample-T160) exhibit η = 5.16% with VOC = 0.609 V, JSC = 13.22 mAcm−2 and FF = 60.9. This enhancement is due to effective light scattering between the 3D flowers. Further T170 sample shows slightly higher efficiency (5.32%). However, the 3D dendritic urchin samples (T180 and T190 hollow urchin) exhibit drastic enhancement in current density from 12.83 mAcm−2 to 12.98 mAcm−2 and 17.17 mAcm−2 respectively. The power conversion efficiency of the T190 sample is 7.16% with VOC = 0.612 V and FF = 64.7. This morphology offers higher surface area and unique morphology that facilitates scattering of light and effective light harvesting.
The incident-photon-to-current conversion efficiency (IPCE) spectra would offer detailed information on the effective light harvesting capability of the DSSCs based on TiO2 samples. Figure 6 shows the IPCE spectra as a function of wavelength for T100 ( ) and T190 (
) and T190 ( ) samples. The IPCE spectrum of N719-Dye loaded T190 device shows maximum absorption at approximately 535 nm, which is attributed to the contribution of effective dye loading. The 3D dendritic hollow urchin sample exhibits 83% IPCE at 535 nm while T100 sample shows only ~42% IPCE. The T190 sample shows a higher IPCE from 350 nm to 700 nm wavelength range than T100 sample, which correlate well with increased photocurrent density values.
) samples. The IPCE spectrum of N719-Dye loaded T190 device shows maximum absorption at approximately 535 nm, which is attributed to the contribution of effective dye loading. The 3D dendritic hollow urchin sample exhibits 83% IPCE at 535 nm while T100 sample shows only ~42% IPCE. The T190 sample shows a higher IPCE from 350 nm to 700 nm wavelength range than T100 sample, which correlate well with increased photocurrent density values.
The Jsc values have been estimated by using following IPCE equation28

where h is Planck's constant (Js−1), c is the speed of light in vacuum (ms−1) and λ1 and λ2 (nm) are the limits of the active spectrum of the device. P incident photon flux density at wavelength λ. The external quantum efficiency (EQE) of the hydrothermally grown TiO2 nanostructures devices are defined as the ratio of the collected electrons to the incident photons. The above equation can be modified as follows

Solving above integration of both the IPCE spectra with the AM1.5 G solar photon flux yields a current density of 8.42 mAcm−2 and 17.97 mAcm−2 for T100 and T190 samples respectively, which is in well agreement with the measured photocurrent density from J-V curves.
Overall, the enhancement of power conversion efficiency for the 3D dendrites and 3D dendritic hollow urchin like nanostructures compared with other nanoparticulate, nanorods, nanoflowers could be attributed to the excellent surface area, effective dye loading, effective light scattering and harvesting into the 3D nanostructures.
Discussion
We have developed a novel and facile approach for exotic nanostructures of TiO2 by simple, cost effective and surfactant free hydrothermal route for the first time using tetrabutyl titanate (TBT) precursor. The nano-morphologies were tuned from clusters of nanoparticles to 1D tetragonal nanorods pillars to 3D dendrites to 3D dendritic hollow urchin architectures by controlling preparative parameters. The deposited samples show different nanostructures containing nanorod assembly with average ~30 nm in diameter. This unique morphology has been created with an adequate combination of temperature and precursor medium. Based on these 3D dendrites and 3D dendritic hollow urchin containing bunch of aligned nanorod with 30 nm diameter show 5.32% and 7.16% conversion efficiency respectively, which is much higher than clusters of nanoparticles as well as nanorods pillars. Such type of hybrid nanostructured assembly will be also helpful for photocatalysis, lithium ion batteries, QDSSCS, hybrid polymer solar cell (HPSC) and efficient flexible dye sensitized solar cell. This approach may pave the way to synthesize better and efficient TiO2 electrodes for respective applications at low cost.
Methods
In a typical synthesis process, 0.5 ml Titanium (IV) butoxide (C16H36O4Ti) (Aldrich, 97%) (TBT) was dissolved in equal volume of concentrated HCl (37% Sigma Aldrich) and distilled water by magnetic stirring. The clear and transparent solution was transferred into a Teflon-lined stainless steel autoclave with a total volume of 25 mL. The fluorine doped tin oxide (FTO) coated glass substrate was then immersed into the solution parallel to the Teflon wall. The sealed autoclave was then kept in a furnace for 3 h at different temperatures. After synthesis, the autoclave was cooled to room temperature naturally. In the present investigation, the hydrothermal synthesis was conducted at various temperatures from 100°C to 190°C. The deposited samples were designated as T100, T110, T120, T130, T140, T150, T160, T170, T180 and T190 for 100°C, 110°C, 120°C, 130°C, 140°C, 150°C, 160°C, 170°C, 180°C and 190°C respectively. The TiO2 deposited FTO substrate was taken out, rinsed with deionized water and allowed to dry in an oven for 30 min. The fresh hydrothermal solution was prepared before each experiment. The surface morphology of the samples were recorded by a field emission scanning electron microscope (FESEM; S-4700, Hitachi). Transmission electron microscopy (TEM) micrographs, selected area electron diffraction (SAED) pattern and high-resolution transmission electron microscopy (HRTEM) images were obtained by TECNAI F20 Philips operated at 200 KV. The TEM sample was prepared by drop casting of ethanolic dispersion of TiO2 samples onto a carbon coated Cu grid. The X-ray diffraction (XRD) measurements were carried out using a D/MAX Uitima III XRD spectrometer (Rigaku, Japan) with Cu Kα line of 1.5410 Å. The elemental information regarding the deposited TiO2 sample was analyzed using an X-ray photoelectron spectrometer (XPS) (VG Multilab 2000-Thermo Scientific, USA, K-Alpha) with a multi-channel detector, which can endure high photonic energies from 0.1 to 3 keV.
The deposited photoelectrodes were further treated with aqueous TiCl4 solution followed by annealing at 450°C. The TiCl4 treated TiO2 photoelectrodes were subsequently soaked in ethanolic 0.5 mM N719 dye (Dyesol) solution at room temperature for 24 h and then washed carefully in ethanol. A compact and sealed dye sensitized solar cell (DSSC) was fabricated using a standard two electrode configuration, comprising dye loaded Glass/FTO/TiO2 (with an active surface area of 0.25 cm2) as the photoanode and platinum coated FTO as the counter electrode, which is sealed with the working electrode using a thermoplastic (~1 μm). The Pt/FTO counter electrodes were prepared by commercial Pt-paste (Solaronix) using doctor blade technique. The deposited Pt/FTO substrate annealed at 450°C for 30 min in air. The iodide-based electrolyte (Dyesol) was used as the redox electrolyte and injected into the interelectrode space from the counter electrode side through a pre-drilled hole. The cells were illuminated using a solar simulator at AM 1.5 G for 10 s, where the light intensity was adjusted with an NREL-calibrated Si solar cell with a KG-5 filter to 1 sun intensity (100 mW cm−2). The incident-photon-to-current conversion efficiency (IPCE) spectra were measured as a function of wavelength from 300 nm to 1200 nm on the basis of a Spectral Products DK240 monochromator.
References
Fujishima, A. & Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 238, 37–38 (1972).
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Robel, I., Kuno, M. & Kamat, P. V. Size-Dependent Electron Injection from Excited CdSe Quantum Dots into TiO2 Nanoparticles. J. Am. Chem. Soc. 129, 4136–4137 (2007).
Mali, S. S. et al. PbS quantum dot sensitized anatase TiO2 nanocorals for quantum dot-sensitized solar cell applications. Dalton Trans. 41, 6130–6136 (2012).
Mali, S. S. et al. Effective light harvesting in CdS nanoparticle-sensitized rutile TiO2 microspheres. Electrochim. Acta 90, 666–672 (2013).
Bai, H., Liu, Z. & Sun, D. D. A lithium-ion anode with micro-scale mixed hierarchical carbon coated single crystal TiO2 nanorod spheres and carbon spheres. J. Mater. Chem. 22, 24552–24557 (2012).
Liu, B. & Aydil, E. S. Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 131, 3985–3990 (2009).
Ding, K. et al. Facile Synthesis of High Quality TiO2 Nanocrystals in Ionic Liquid via a Microwave-Assisted Process. J. Am. Chem. Soc. 129, 6362–6363 (2007).
Guo, W. et al. Rectangular Bunched Rutile TiO2 Nanorod Arrays Grown on Carbon Fiber for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 134, 4437–4441 (2012).
Liao, J.-Y., Lei, B.-X., Kuang, D.-B. & Su, C.-Y. Tri-functional hierarchical TiO2 spheres consisting of anatase nanorods and nanoparticles for high efficiency dye-sensitized solar cells. Energy Environ. Sci. 4, 4079–4085 (2011).
Liao, J.-Y., Lei, B.-X., Chen, H.-Y., Kuang, D.-B. & Su, C.-Y. Oriented hierarchical single crystalline anatase TiO2 nanowire arrays on Ti-foil substrate for efficient flexible dye-sensitized solar cells. Energy Environ. Sci. 5, 5750–5757 (2012).
Wu, W.-Q. et al. Hydrothermal Fabrication of Hierarchically Anatase TiO2 Nanowire arrays on FTO Glass for Dye-sensitized Solar Cells. Sci. Rep. 3, 1352–1357 (2013).
Lv, M. et al. Optimized porous rutile TiO2 nanorod arrays for enhancing the efficiency of dye-sensitized solar cells. Energy Environ. Sci. 6, 1615–1622 (2013).
Mali, S. S. et al. Hydrothermal synthesis of rutile TiO2 nanoflowers using Brønsted Acidic Ionic Liquid [BAIL]: Synthesis, characterization and growth mechanism. CrystEngComm 14, 1920–1924 (2012).
Mali, S. S., Betty, C. A., Bhosale, P. N. & Patil, P. S. Hydrothermal synthesis of rutile TiO2 with hierarchical microspheres and their characterization. CrystEngComm 13, 6349–6351 (2011).
Mali, S. S. et al. Efficient dye-sensitized solar cells based on hierarchical rutile TiO2 microspheres. CrystEngComm 14, 8156–8161 (2012).
Hosono, E., Fujihara, S., Kakiuchi, K. & Imai, H. Growth of Submicrometer-Scale Rectangular Parallelepiped Rutile TiO2 Films in Aqueous TiCl3 Solutions under Hydrothermal Conditions. J. Am. Chem. Soc. 126, 7790–7791 (2004).
Berhe, S. A., Nag, S., Molinets, Z. & Youngblood, W. J. Influence of Seeding and Bath Conditions in Hydrothermal Growth of Very Thin (20 nm) Single-Crystalline Rutile TiO2 Nanorod Films. ACS Appl. Mater. Interfaces 5, 1181–1185 (2013).
Cheng, H. M., Ma, J. M., Zhao, Z. G. & Qi, L. M. Hydrothermal Preparation of Uniform Nanosize Rutile and Anatase Particles. Chem. Mater. 7, 663–671 (1995).
Kumar, A., Madaria, A. R. & Zhou, C. W. Growth of Aligned Single-Crystalline Rutile TiO2 Nanowires on Arbitrary Substrates and Their Application in Dye-Sensitized Solar Cells. J. Phys. Chem. C 114, 7787–7792 (2010).
Mali, S. S. et al. Single-step synthesis of 3D nanostructured TiO2 as a scattering layer for vertically aligned 1D nanorod photoanodes and their dye-sensitized solar cell properties. CrystEngComm 15, 5660–5667 (2013).
Yu, J. G., Fan, J. J. & Lv, K. L. Anatase TiO2 nanosheets with exposed (001) facets: improved photoelectric conversion efficiency in dye-sensitized solar cells. Nanoscale 2, 2144–2149 (2010).
Wu, X., Chen, Z., Lu, G. Q. & Wang, L. Solar cells: nanosized anatase TiO2 single crystals with tunable exposed (001) facets for enhanced energy conversion efficiency of dye-Sensitized Solar Cells. Adv. Funct. Mater. 21, 4166–4166 (2011).
Jung, M. H., Chu, M. J. & Kang, M. G. TiO2 nanotube fabrication with highly exposed (001) facets for enhanced conversion efficiency of solar cells. Chem. Commun. 48, 5016–5018 (2012).
Sun, Z. et al. Rational Design of 3D Dendritic TiO2 Nanostructures with Favorable Architectures. J. Am. Chem. Soc. 133, 19314–19317 (2011).
Sun, Z. et al. Continually adjustable oriented 1D TiO2 nanostructure arrays with controlled growth of morphology and their application in dye-sensitized solar cells. CrystEngComm 14, 5472–5478 (2012).
Sun, Z., Kim, J. H., Zhao, Y., Attard, D. & Dou, S. X. Morphology-controllable 1D–3D nanostructured TiO2 bilayer photoanodes for dye-sensitized solar cells. Chem. Comm. 49, 966–968 (2013).
Dennler, G., Scharber, M. C. & Brabec, C. J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 21, 1323–1338 (2009).
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2009-0094055).
Author information
Authors and Affiliations
Contributions
S.S.M. & C.K.H. contributed to the conception and design of the experiments, analysis of the data and writing the paper. S.S.M. carried out all experiments and wrote the paper. S.S.M., H.J.K. and C. S. S. performed J-V, IPCE measurement, calculations and analyzed data. P.S.P. and J.H.K. participated in the scientific discussion and valuable suggestions during the course of this manuscript. All authors discussed the results and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Surfactant free most probable TiO2 nanostructures via hydrothermal and its dye sensitized solar cell properties
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mali, S., Kim, H., Shim, C. et al. Surfactant free most probable TiO2 nanostructures via hydrothermal and its dye sensitized solar cell properties. Sci Rep 3, 3004 (2013). https://doi.org/10.1038/srep03004
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03004
This article is cited by
-
Biosynthesis, characterization and optimization of TiO2 nanoparticles by novel marine halophilic Halomonas sp. RAM2: application of natural dye-sensitized solar cells
Microbial Cell Factories (2023)
-
Solvent assisted evolution and growth mechanism of zero to three dimensional ZnO nanostructures for dye sensitized solar cell applications
Scientific Reports (2021)
-
Studying the role of ZnO nanostructure photoanodes for improving the photovoltaic performance of CdSe QDSSCs
Journal of Materials Science: Materials in Electronics (2021)
-
Effect of nanoporous In2O3 film fabricated on TiO2-In2O3 photoanode for photovoltaic performance via a sparking method
Journal of Solid State Electrochemistry (2018)
-
Novel synthetic route for the synthesis of ternary Cd(SSe) photoelectrode and their photoelectrochemical application
Journal of Materials Science: Materials in Electronics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.








 ) nanoparticulate and T190 (
) nanoparticulate and T190 ( ) 3D dendritic hollow urchin samples.
) 3D dendritic hollow urchin samples.