Abstract
Understanding the genetics behind adaptation and reproductive isolation contributes to our knowledge about how biodiversity is created and maintained. Host races of phytophagous insects are host-associated ecotypes and have been considered as candidates for ecological speciation, but very little is known about the genetic backgrounds of host adaptations. A leaf-mining moth, Acrocercops transecta, consists of Juglans- and Lyonia-associated host races. This study assesses the genetic bases of oviposition preference and larval performance using F1, F2 and backcross hybrids between the two host races. Segregation patterns in the hybrid generations revealed that larval performance on Juglans is dominant, but oviposition preference for Lyonia is dominant. This result indicates that genetic components introgressed from the Lyonia race are removed from the Juglans race even though hybrid larvae are viable on Juglans. Thus, simple genetic controls with contrasting dominance directions in host-adaptation traits function as barriers to prevent a fusion of host races.
Similar content being viewed by others
Introduction
Adaptation to a novel environment often requires the evolution of multiple traits and hybridization between ecologically divergent taxa could produce maladaptive phenotype combinations, resulting in isolation barriers1,2,3,4,5,6. In phytophagous insects, a precise combination of preference (e.g., ovipositing female preference) and performance (e.g., larval tolerance to secondary compounds) for particular host plants is crucial because a new host plant can be incorporated into an insect's diet only if adults accept it for oviposition and if the larvae are able to complete their development on it7. In most cases, preferences and performances in phytophagous insects are under genetic control8. Thus, differences in the mode of inheritance between these two traits may result in isolating barriers between host races.
To address the genetic mechanisms preventing the fusion of host races, I studied a leaf-mining moth, Acrocercops transecta (Gracillariidae), which consists of Juglans (Juglandaceae)- and Lyonia (Ericaceae)-associated host races (see Supplementary Background Text for details). The two host races clearly differ in the host preferences of ovipositing females and larval performances on host plants but mate readily in the laboratory, producing fertile hybrids9. Because the resistance to Lyonia is completely recessive to resistance to Juglans, the F1 hybrid larvae can survive only when they feed on Juglans9, indicating that gene flow should be directed from the Lyonia race to the Juglans race. However, because F1 larvae exhibit high viability on Juglans9, gene flow from the Juglans race to the Lyonia race is also possible if eclosed F1 hybrid males mate with females of the Lyonia race. The Juglans and Lyonia races are often sympatric in the wild and there is no phenological or host-associated premating isolation between them10,11. Indeed, gene flow has occurred in both directions between the two host races in the wild12,13. Thus, there should be postmating genetic mechanisms that maintain the differences between the two host races even in the face of gene flow.
Maladaptive alleles introgressed through hybridization are likely to be eliminated from respective host races, but the strength and extent of purifying selection against the alleles depend on the mode of inheritance of each locus. Although a previous study has revealed the direction of dominance of larval performance9, the segregation patterns in F2- and backcross-hybrid larvae are still unknown. Further, the oviposition preferences in F1-, F2- and backcross-hybrid females are also still unknown. Thus, detailed observations of phenotypes in both oviposition preference and larval performance in hybrid generations are crucial to infer the genetic bases of host adaptation and to evaluate their contribution to an isolating barrier between the two host races of A. transecta.
To investigate the genetic bases of oviposition preference and larval performance, two sympatric and five allopatric host-associated populations (Juglans race: Sendai, Sapporo and Yamagata; Lyonia race: Sendai, Okazaki, Kyoto and Kirishima; see Supplementary Fig. S1 online) in Japan were used for the experiments. First, F1, F2 and all combinations of backcrosses were established using the sympatric populations (Sendai) (see Supplementary Fig. S2A-H online). Next, I further assessed the segregation patterns of oviposition preference using backcrosses between Juglans females and F1 hybrid males (J♀ x JL♂ backcross) that were established from Sapporo (Juglans race) and Kyoto (Lyonia race) populations (see Supplementary Fig. S2I online). Finally, segregation patterns of larval performance were further assessed using backcrosses between F1 hybrid females and Lyonia males and vice versa (JL♀ x L♂ and L♀ x JL♂ backcrosses, respectively). I used Yamagata (Juglans race) and Okazaki and Kirishima (Lyonia race) populations for establishing the backcrosses (see Supplementary Fig. S2J, K online).
The goal of this study is to reveal the modes of inheritance of adaptive traits and to uncover the genetic mechanisms causing isolating barriers between ecologically divergent taxa.
Results
Oviposition preferences in F1 hybrid females
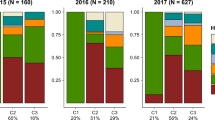
Reciprocal hybrids (JL and LJ) were assessed in this experiment. F1 hybrid females significantly preferred to oviposit on Lyonia rather than Juglans regardless of the direction of crosses, except for two females from JL crosses that laid more eggs on Juglans than on Lyonia (JL t = 7.471, df = 38, P = 5.727e-9; LJ t = 4.225, df = 4, P = 1.343e-2; paired t-test) (Fig. 1A–D). Because all F1 hybrids fed on Juglans during their larval stages, the results indicate that the oviposition preference of adult females is determined not by larval experiences but by genetic factors. The results also indicate that the loci determining oviposition preference are located on autosomes and that the preference for Lyonia is dominant over that for Juglans. Thus, I used only JL F1 hybrids for establishing F2 and backcross hybrids in the subsequent experiments.
Oviposition preference in F1, F2 and backcross hybrids.
Mean values and standard deviations for the numbers of eggs laid (A, C, E, G, I, K, L, M, O, Q, R) and the individual ovipositing female preference (B, D, F, H, J, N, P) in each of the F1, F2 and backcross hybrids. In J x JL backcrosses, Juglans- or Lyonia-type females are separately illustrated because there were two types of females with regard to oviposition preference (K, L, Q, R).
Oviposition preferences in F2 and backcross hybrid females
Assuming that oviposition preference is determined by a single-locus, two-allele system and that the Lyonia-preferring allele is dominant to the Juglans-preferring allele, the expected segregation ratios are 1:3 (prefer to oviposit on Juglans:Lyonia) in F2, 0:1 in JL x L and L x JL and 1:1 in J x JL (JL x J backcross is lethal, see below). In each of the pooled JL x L, L x JL and J x JL backcrosses in the Sendai population, hybrid individuals segregated in the expected ratio (Fig. 1G-N; Table 1A). All females preferred to oviposit on Lyonia in the JL x L (Sendai) and L x JL (Sendai) backcrosses (JL x L t = 9.798, P = 1.202e-7, df = 14; L x JL t = 4.757, P = 1.828e-4, df = 17; paired t-test) (Fig. 1G, H, M, N). In the J x JL (Sendai) backcross, 95 females preferred to oviposit on Juglans and 87 preferred Lyonia, although three females laid eggs evenly on Juglans and Lyonia (Fig. 1J). For the 95 females that preferred Juglans, the mean number of eggs deposited on Juglans was significantly larger than that on Lyonia (paired-t test, t = 13.1666, P = 2.2e-16, df = 94) and the 87 females that preferred Lyonia deposited significantly more eggs on Lyonia (paired t-test, t = 10.9945, P = 2.2e-16, df = 86) (Fig. 1K, L). These results are consistent with the hypothesis of Mendelian inheritance, with the dominance of a Lyonia-preferring allele in the preference gene.
However, F2 (Sendai) and the J x JL backcross (Sapporo x Kyoto) demonstrated biased segregation ratios (Fig. 1F, P; Table 1A). One possible hypothesis to explain the deviation from the expected ratio in F2 hybrids is that the preference gene is physically linked to performance genes. Because all F2 hybrid larvae were reared on Lyonia because of the oviposition preference of F1 hybrid females and because the resistance to Lyonia is completely recessive to resistance to Juglans9, approximately three-quarters of F2 hybrid larvae failed to survive on Lyonia (Fig. 2; Table 1B). Thus, given the linkage between preference and performance loci, all F2 hybrids possessing a Juglans-preferring allele died on Lyonia. In contrast, the biased segregation pattern in the J x JL backcross (Sapporo x Kyoto) is difficult to explain. One of the possible reasons for this deviation is the experimental condition: a shortage of fresh Lyonia leaves may have inhibited the oviposition of females because the experiments were conducted during winter and I used Lyonia plants maintained in a greenhouse. Alternatively, oviposition preference may be governed by several genes or influenced by maternal effects (e.g., symbionts) in the Sapporo population. There were much fewer females assessed in the J x JL backcross (Sapporo x Kyoto) than in the J x JL backcross (Sendai), so additional experiments with a larger sample size of J x JL backcross (Sapporo x Kyoto) females could reveal the factors for this deviation. However, what can be concluded is that Lyonia-preference is dominant to Juglans-preference and that a few loci control oviposition preference.
Hatchability and viability until the second stadium in F1, F2, backcross hybrids and pure Juglans or Lyonia races.
For viability, a significant difference was found among crosses (F8, 41 = 82.09, P < 2.2e-16, one-way ANOVA). Thus, post hoc Tukey-Kramer HSD pairwise comparisons were performed between crosses. Different letters indicate significant differences (P < 0.05, Tukey-Kramer HSD test).
Larval performances in F2 and backcross hybrid
Performance on Juglans is dominant over that on Lyonia9. Thus, assuming that larval performance is determined by a single-locus, two-allele system, the expected segregation ratios are 1:3 (survive:die on Lyonia) in F2, 0:1 (survive:die on Lyonia) in JL x J, 1:1 (survive:die on Lyonia) in JL x L and L x JL and 1:0 (survive:die on Juglans) in J x JL backcrosses. All segregation patterns in F2 and backcross hybrids supported the hypothesis except the J x JL backcross hybrids (Table 1B). However, the viability of the J x JL backcross was not significantly different from that of the control crosses (Fig. 2, see Supplementary Table S1 online for detailed statistical results), suggesting that the slightly reduced viability was not due to the lack of the resistance to Juglans but due to accidental mortality. Thus, the present results indicate that larval performance is governed by a single-locus, two-allele system with complete dominance of resistance to Juglans. In addition, the hatchability of the eggs from every cross combination was higher than 90%. There were no significant differences in hatchability between pure races and hybrids (F8, 41 = 0.7583, P = 0.6406) (Fig. 2), indicating that there was not intrinsic reproductive isolation between the two host races.
Discussion
The present results provide evidence that the difference in both ovipositing female preference and larval performance between host races of A. transecta is each mainly determined by a single-locus, two-allele system with dominance, respectively. However, a few females showed an intermediate oviposition preference and a biased segregation ratio in the J x JL backcross (Sapporo x Kyoto), implying the existence of modifier genes or maternal effects in host preference. The directions of dominance for female preference and larval performance were opposite, indicating that preference and performance are under different genetic controls.
The present findings have implications for the mechanism that prevent the fusion of the two host races even when gene flow occurs. The allele for resistance to Juglans is dominant. Thus, F1 larvae could survive if Juglans females mate with Lyonia males, resulting in asymmetrical gene flow from the Lyonia race to the Juglans race (Fig. 3). However, eclosed F1 females avoid ovipositing on Juglans because of the expression of the Lyonia-preferring allele. This result indicates that genetic components that introgressed from the Lyonia race were removed from the Juglans race (Fig. 3). Thus, the differences in the direction of dominance between preference and performance loci themselves function as a barrier to prevent the fusion of the two host races. A physical linkage between the preference and performance loci would make removal of the genomic components responsible for the resistance to Lyonia from the Juglans race more easily and this possibility should be assessed in future mapping studies.
Summary of the genetic mechanisms that prevent fusion of the two host races in A. transecta.
(a) F1 hybrid eggs from crosses between Lyonia females and Juglans males are deposited on Lyonia. However, F1 hybrid larvae cannot survive on Lyonia because of an expression of the dominant Juglans-resistance allele. Thus, alleles for Juglans resistance and Juglans preference are eliminated from the Lyonia race immediately. (b) F1 hybrid larvae from crosses between Juglans females and Lyonia males can develop to adulthood on Juglans, but no eclosed adult females prefer to oviposit on Juglans because of an expression of the dominant Lyonia-preference allele. Even if F1 males mate with Juglans females, approximately half of the resulting female backcross offspring will avoid ovipositing on Juglans as adults. Therefore, alleles for Lyonia preference are sequentially removed from the Juglans race.
The present study also indicates that the genetics of host adaptation in A. transecta contributes to the reproductive isolation of the two host races. If the females of F1 hybrids mate with males of the Juglans race, the females oviposit all eggs on Lyonia, but no backcross larvae can survive on Lyonia (Fig. 3). Similarly, even if the F1 hybrids mate with Lyonia females or males, only half of the backcross hybrids would express the recessive trait, resulting in reduced viability on Lyonia (Fig. 3). Therefore, hybrids suffer from incongruent phenotypes for preference and performance because of the opposite directions of dominance. This incongruent dominance could be a prime barrier against gene flow between the two host races of A. transecta.
Although the genetics of ecological adaptations have received much attention in the study of speciation and species differences14, the implications of such studies for understanding how ecological speciation occurs are unclear. One reason for this lack of clarity is that empirical data for the genetics of ecological adaptation vary among species (e.g., governed by autosomal loci or sex-linked genes, few or many genes, genes of small or large effect, or genes with dominance, epistatic interactions or no dominance)15. However, the present results and a growing number of studies have demonstrated that phytophagous insects have different genetic bases between preference and performance with different modes of inheritance7,8,16,17,18,19,20,21,22. This implies that hybrids are likely to express different and often functionally incompatible, phenotypes for preference and performance traits. Indeed, Forister21 and Nygren et al.22 have revealed that the differences in dominance directions and sex-linkage for preference and performance loci break the correlation between the two traits in F1 hybrids, respectively. The present study further demonstrates that differences in dominance directions between preference and performance loci lead to ecological incompatibilities in subsequent backcross generations (Fig. 3).
In phytophagous insects, the growing larvae often complete their entire development on a single host plant individual. Hence, a set of genes that function well together on one host is crucial and could be an expected evolutionary outcome23,24. Therefore, hybrids with incompatible host-adaptation genes are likely to be under strong disruptive selection as indicated by the Bateson-Dobzhansky-Muller (BDM) model for postzygotic genomic incompatibilities25,26,27,28. Therefore, the different modes of inheritance for selected traits may be an important postzygotic isolation mechanism in phytophagous insects. Although these mechanisms have rarely been emphasized, they may prove to be a major factor promoting host-race formation and consequently the high specialization and species diversity observed in phytophagous insects.
Methods
Moth collection and rearing
I collected mined leaves containing larvae from the host plants, Juglans ailanthifolia or J. regia and Lyonia ovalifolia and maintained them in the laboratory29. Two sympatric (Sendai, Japan [38°15′N, 140°49′E]) and five allopatric host-associated populations (Juglans race: Sapporo [43°07′N, 141°34′E] and Yamagata [38°33′N, 140°49′E]; Lyonia race: Okazaki [34°94′N, 137°17′E], Kyoto [35°02′N, 135°79′E] and Kirishima [31°86′N, 130°77′E]; see Supplementary Fig. S1 online) were used for the experiments. All moths used for parental crosses were collected from the field as larvae, together with the leaves on which they were mined. Collected larvae were reared on their respective mined leaves in the laboratory following the methodology described by Ohshima29. All rearing and experimental work was conducted at 25 or 27°C under the photoperiod of a 16:8 L:D cycle with 50–70% relative humidity.
Eclosed adult moths were used for crossing and all crosses were single-pair crosses. A single pair of virgin moths was transferred with an aspirator to one plastic vial (a 118-mm long, 28-mm diameter centrifuge tube) for pairing. After mating, each female that had started oviposition was individually introduced into a plastic container (120 x 200 x 50, 125 x 225 x 50 or 175 x 260 x 50 mm, depending on the leaf size). Three to six leaves of a host plant were placed in the container to obtain F1 and backcross hybrid eggs. All F1 hybrid larvae were maintained on Juglans because F1 hybrid larvae can survive only when they feed on Juglans (i.e., resistance to Juglans is dominant over that to Lyonia)9.
The Juglans and Lyonia leaves used in the present experiments were collected directly from the study localities or from plants that had been transplanted from the localities to greenhouses and randomly used for each trial. I used two Juglans species, J. ailanthifolia and J. regia, for the present experiments, but there were no significant differences in either ovipositing female preference or larval performance between J. ailanthifolia and J. regia (Ohshima pers. obs.).
Establishment of F1 hybrids for assessing oviposition preference
The sympatric Sendai populations were used for assessing oviposition preference in F1 hybrid females. Ten broods were established for the JL F1 hybrids (crosses between Juglans females and Lyonia males) and four broods were established for the LJ F1 hybrids (crosses between Lyonia females and Juglans males) (see Supplementary Fig. S2A online). Because Lyonia females did not normally oviposit on Juglans leaves and it was impossible to transfer eggs to other leaves, I tried to induce the Lyonia females to mis-oviposit on Juglans leaves by placing a single Juglans leaf over Lyonia leaves in the plastic container. For this reason, a small number of F1 adult females were available in the LJ F1 hybrids (a total of five).
Establishment of F2 and backcross hybrids for assessing larval performance
First, F2 and all combinations of backcrosses were established using the sympatric populations in Sendai (see Supplementary Fig. S2B-F online). Five broods were established for the JL x J backcross (crosses between F1 females from JL crosses and Juglans males) (Fig. S2B). Similarly, five broods of the JL x L backcross (crosses between F1 females and Lyonia males) (Fig. S2C), J x JL (crosses between Juglans females and F1 males) (Fig. S2D), L x JL (crosses between Lyonia females and F1 males) (Fig. S2E) and F2 generations, JL x JL (crosses between F1 females and males from JL crosses) (Fig. S2F) were prepared. I also prepared JJ (crosses between Juglans females and males) and LL (crosses between Lyonia females and males) crosses as controls using the Sendai populations (five broods each). Due to the strong host preference of ovipositing females, larvae of backcross hybrids from J x JL and a control cross JJ were exclusively maintained on Juglans. Similarly, hybrid larvae from JL x J, JL x L, L x JL, JL x JL (F2 hybrids) and LL (control) crosses were maintained on Lyonia (F1 hybrid females exclusively prefer to oviposit on Lyonia. See the main text). These experiments were conducted from 2003 to 2005.
I further assessed the segregation patterns of larval performance in backcrosses between F1 hybrid females and Lyonia males and vice versa (JL x L and L x JL backcrosses) using three allopatric populations (Juglans race: Yamagata; Lyonia race: Okazaki and Kirishima). Six and nine broods were established for the JL x L and L x JL backcrosses, respectively (see Supplementary Fig. S2J, K online). These experiments were conducted in 2009.
Establishment of F2 and backcross hybrids for assessing oviposition preference
For F2 and backcross hybrids, larvae of each cross combination used in the assessment of larval performance were maintained until adulthood to investigate female oviposition preference, except for JL x J backcrosses (because they are lethal). An additional 22 broods were established for the J x JL backcross and five broods were established for F2 hybrids (see Supplementary Fig. S2G, H online). These experiments with the Sendai populations were conducted from 2003 to 2005 and in 2010.
I further assessed the segregation patterns of oviposition preference using a J x JL backcross that originated from Sapporo (Juglans race) and Kyoto (Lyonia race) populations. Thirteen broods were established for these crosses (see Supplementary Fig. S2I online) in 2007.
Assessing larval performance in F2 and backcross generations
A previous study revealed that hybrid larvae with a maladaptive performance genotype could not survive until the second stadium on Lyonia9. For this reason, I recorded the numbers of hatched eggs and the viability of larvae that reached the second stadium as an index of larval performance.
There was no observed heterogeneity of segregation patterns among broods within a given category of mating (G-test, JL x JL F2 [Sendai] G = 6.995, P = 0.1361, df = 4; JL x J backcross [Sendai] G = 0, P = 1, df = 4; JL x L backcross [Sendai] G = 5.497, P = 0.2399, df = 4; J x JL backcross [Sendai] G = 7.624, P = 0.1063, df = 4; L x JL backcross [Sendai] G = 2.839, P = 0.5850, df = 4; JL x L backcross [Yamagata x Okazaki x Kirishima] G = 8.912, P = 0.1126, df = 5; L x JL backcross [Yamagata x Okazaki x Kirishima] G = 1.529, P = 0.9922, df = 8). Therefore, the results are presented for pooled data.
Assessing oviposition preference in F1, F2 and backcross generations
Eclosed F1, F2 and backcross females were crossed with males from their own generation (e.g., F2 females crossed with F2 males) for testing host preference and all crosses were single-pair crosses. A single pair of virgin moths was transferred with an aspirator to one plastic vial (a 118-mm long, 28-mm diameter centrifuge tube) for pairing. After mating, each female that had started oviposition was individually introduced into a plastic container (100 x 100 x 50 mm). One pair of fresh young leaves of Juglans and Lyonia were placed in the container. Similar-sized leaves were chosen. In this container, ovipositing females were allowed to select leaves for oviposition for 24 h after transfer and the number of eggs deposited on each leaf was counted.
There was no observed heterogeneity of segregation patterns among broods within a given category of mating (G-test, JL F1 G = 4.803, P = 0.8511, df = 9; LJ F1 G = 0, P = 1, df = 3; JL x JL F2 [Sendai] G = 0, P = 1, df = 9; JL x L backcross [Sendai] G = 0, P = 1, df = 4; J x JL backcross [Sendai] G = 28.86, P = 0.3174, df = 26; L x JL backcross [Sendai] G = 0, P = 1, df = 4; J x JL backcross [Sapporo x Kyoto] G = 13.21, P = 0.3539, df = 12). Therefore, results are presented for pooled data. Females were considered to be a Juglans (or Lyonia) type if more than half of the eggs they laid were on Juglans (or Lyonia).
Statistical analyses
Egg hatchability and larval viability until the second stadium of the F2, backcross and control generations were transformed to arcsine square roots to satisfy the requirement of normality and the transformed values were analyzed using one-way ANOVA. For viability, a significant difference was found among crosses (F8, 41 = 82.09, P < 2.2e−16). Thus, post hoc Tukey-Kramer HSD (α = 0.05) pairwise comparisons were performed between crosses. The numbers of eggs deposited on the respective leaves in the oviposition preference experiments were analyzed by a paired t-test. The hypotheses of a single-locus, two-allele system with complete dominance for the segregation patterns of larval performance and of ovipositing female preference in F2 and backcross generations were analyzed by goodness-of-fit (G-test)30. All statistical tests were performed using R 2.11.031.
References
Levene, H. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87, 331–333 (1953).
Bush, G. Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera, Tephritidae). Evolution 23, 237–251 (1969).
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ Press, Oxford, 2000).
Schluter, D. Ecology and the origin of species. Trends Ecol. Evol. 16, 372–380 (2001).
Fry, J. D. Multilocus models of sympatric speciation: Bush versus Rice versus Felsenstein. Evolution 57, 1735–1746 (2003).
Futuyma, D. J. Evolution (Sinauer, Sunderland, 2005).
Sezer, M. & Butlin, R. K. The genetic basis of oviposition preference differences between sympatric host races of the brown planthopper (Nilaparvata lugens). Proc. R. Soc. Lond. B Biol. Sci. 265, 2399–2405 (1998).
Matsubayashi, K., Ohshima, I. & Nosil, P. Ecological speciation in phytophagous insects. Entomol. Exp. Appl. 134, 1–27 (2010).
Ohshima, I. Host race formation in the leaf-mining moth Acrocercops transecta (Lepidoptera: Gracillariidae). Biol. J. Linn. Soc. 93, 135–145 (2008).
Kumata, T. Kuroko, H. & Ermolaev, V. P. Japanese species of the Acrocercops-group (Lepidoptera: Gracillariidae), part I. Ins. Matsum. N. S. 38, 1–111 (1988).
Ohshima, I. Host-associated pre-mating reproductive isolation between host races of Acrocercops transecta: mating site preferences and effect of host presence on mating. Ecol. Entomol. 35, 253–257 (2010).
Ohshima, I. & Yoshizawa, K. Differential introgression causes genealogical discordance in host races of Acrocercops transecta (Insecta: Lepidoptera). Mol. Ecol. 19, 2106–2119 (2010).
Ohshima, I. & Yoshizawa, K. The utility of indels in population genetics: the Tpi intron for host race genealogy of Acrocercops transecta (Insecta: Lepidoptera). Mol. Phylogenet. Evol. 59, 469–476 (2011).
Coyne, J. A. & Orr, H. A. Speciation (Sinauer, Sunderland, 2004).
Rundle, H. D. & Nosil, P. Ecological speciation. Ecol. Lett. 8, 336–352 (2005).
Sezer, M. & Butlin, R. K. The genetic basis of host plant adaptation in the brown planthopper (Nilaparvata lugens). Heredity 80, 499–508 (1998).
Jones, C. D. The genetic basis of Drosophila sechellia's resistance to a host plant toxin. Genetics 149, 1899–1908 (1998).
Jones, C. D. The genetics of adaptation in Drosophila sechellia. Genetica 123, 137–145 (2005).
Matsuo, T., Sugaya, S., Yasukawa, J., Aigaki, T. & Fuyama, Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biology 5, e118 (2007).
Hora, K. J., Roessingh, P. & Menken, S. B. J. Inheritance and plasticity of adult host acceptance in Yponomeuta species: implications for host shifts in specialist herbivores. Entomol. Exp. Appl. 115, 271–281 (2005).
Forister, M. L. Independent inheritance of preference and performance in hybrids between host races of Mitoura butterflies (Lepidoptera: Lycaenidae). Evolution 59, 1149–1155 (2005).
Nygren, G. H., Nylin, S. & Stefanescu, C. Genetics of host plant use and life history in the comma butterfly across Europe: varying modes of inheritance as a potential reproductive barrier. J. Evol. Biol. 19, 1882–1893 (2006).
Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theoret. Pop. Biol. 14, 350–356 (1978).
Thompson, J. N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 47, 3–14 (1988).
Bateson, W. In: Darwin and modern science: Heredity and variation in modern lights (ed. Seward C.) 85–101 (Cambridge Univ Press, Cambridge, 1909).
Dobzhansky, T. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21, 113–135 (1936).
Muller, H. J. & Pontecorvo, G. Recombinants between Drosophila species the F1 hybrids of which are sterile. Nature 146, 199–200 (1940).
Burke, J. M. & Arnold, M. L. Genetics and the fitness of hybrids. Annu. Rev. Genet. 35, 31–52 (2001).
Ohshima, I. Techniques for continuous rearing and assessing host preference of a multivoltine leaf-mining moth, Acrocercops transecta (Lepidoptera: Gracillariidae). Entomol. Sci. 8, 227–228 (2005).
Sokal, R. R. & Rohlf, F. J. Biometry 3rd edn (Freeman, New York, 1995).
R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2009).
Acknowledgements
I thank K. Mizota, R. Deguchi, T. Fukushi, N. Goto, M. Hasebe, Y. Hirabuki, A. Munakata, Y. Saito and the NIBB Model Plant Research Center for use of their laboratory; Y. Ohmizu and H. Yamada for assistance in the rearing of moths; P. Nosil and D. Hembry for comments on the manuscript; T. Kumata, S. Akimoto, N. Fujiyama, N. Kobayashi, K. Matsubayashi, M. Ôhara and K. Yoshizawa for discussion. This work was supported by Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists (16-9250 and 20-5555) and a Grant-in-Aid from JSPS (23870037).
Author information
Authors and Affiliations
Contributions
I.O. conceived and designed the study, collected materials, reared moths with the assistance of those mentioned in the Acknowledgements, collected data, analyzed the data and wrote the paper.
Ethics declarations
Competing interests
The author declares no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ohshima, I. Genetic mechanisms preventing the fusion of ecotypes even in the face of gene flow. Sci Rep 2, 506 (2012). https://doi.org/10.1038/srep00506
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00506
This article is cited by
-
Oviposition stimulants underlying different preferences between host races in the leaf-mining moth Acrocercops transecta (Lepidoptera: Gracillariidae)
Scientific Reports (2022)
-
Host range expansion of a Polygonaceae-associated leaf beetle to an invasive aquatic plant Myriophyllum aquaticum (Haloragaceae)
Arthropod-Plant Interactions (2020)
-
Species diversity of herbivorous insects: a brief review to bridge the gap between theories focusing on the generation and maintenance of diversity
Ecological Research (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.