Abstract
Oxidative stress conditions enhance the production of reactive oxygen species resulting from a variety of stimuli and are associated with various human diseases, including neurodegenerative disorders, inflammation and various cancers. Though such associations have been closely studied using animal models, there has been no in vivo system for monitoring oxidative stress. We have developed an oxidative stress indicator that is dually regulated by induction at the transcriptional level and by protein stabilisation at the post-translational level in Keap1-Nrf2 pathway. In vitro, our indicator elicited an intense and specific signal to oxidative stress among various agents, in a Keap1-Nrf2-dependent manner. Moreover, the transgenic animal expressing the indicator exhibited significant signals upon oxidative stress. These results indicate the usefulness of our system as an indicator of oxidative stress both in vitro and in vivo.
Similar content being viewed by others
Introduction
Oxidative stress conditions enhance the production of reactive oxygen species (ROS) resulting from a variety of stimuli including ionising radiation, exposure to xenobiotics, electrophilic agents and diseases1. Treatment of cells with electrophilic agents usually provokes cellular responses, including transcriptional activation of genes encoding proteins that participate in the defence against oxidative stress.
The Keap1-Nrf2 pathway is a typical cellular defensive system against oxidative stress. Nrf2, a basic region-leucine zipper (bZip) transcription factor, plays a key role in this pathway2,3,4. Under normal conditions, Nrf2 is rapidly degraded by the ubiquitin–proteasome pathway through the association with Keap1 (Kelch-like ECH associating protein 1), a substrate adaptor protein of the Cul3-based ubiquitin E3 ligase complex5,6,7,8. Upon exposure to oxidative or electrophilic stress, reactive cysteine residues in Keap1 are covalently modified, leading to the liberation of Nrf2 from Keap1-mediated degradation. The stabilised Nrf2 is then translocated to the nucleus and interacts with a member of the small Maf family proteins9. This complex activates the transcription of a wide range of cytoprotective genes (HO-1, NQO1, GST, etc.) via a cis-acting DNA element known as the antioxidant/electrophile responsive element (ARE/EpRE)10,11,12.
Importantly, both in in vitro and in vivo models, a great number of studies have shown that the Keap1-Nrf2 pathway is involved in the onset or mitigation of various human diseases, including neurodegenerations like Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis (ALS)13,14. Moreover, recent studies using Nrf2 knockout mice indicate significant, close relationships between Nrf2 and human diseases15,16,17, including acute lung injury or acute inflammation18.
Therefore, studies on oxidative stress in vivo should yield crucial information, from both scientific and medical aspects. However, no oxidative stress indicators which could be used in vivo have been reported. To facilitate in vivo analysis, we developed an indicator for oxidative stress in living cells, named OKD48 (Keap1-dependent Oxidative stress Detector, No-48). Here, we describe the specificity and sensitivity of OKD48 as an oxidative stress indicator and the usefulness of OKD48 transgenic mice.
Results
Design and construction of OKD48, a novel oxidative stress indicator
To design the oxidative stress indicator, we used the dual regulating mechanism in the Keap1-Nrf2 pathway. The designed indicator consists of the following. First, the Nrf2 fragment which contributes to the stress-dependent stabilisation was fused to luciferase. Second, the resulting fusion gene was expressed by oxidative stress inducible promoter (Fig. 1). Under normal conditions, such a fusion gene is not transcriptionally induced and the leaked fusion protein is degraded by Keap1 and therefore no signal is detected. However, under oxidative stress conditions, the fusion gene is induced at the transcriptional level by endogenous Nrf2 and the resulting protein is stabilised at the post-transcriptional level by cancellation of Keap1-mediated degradation. Thus, the dual regulating mechanism produces an intense signal upon oxidative stress (Fig. 1).
Schematic of OKD48 function.
p(3xARE)TKbasal-hNrf2(1-433)GL4-Flag was generated as the OKD48 construct. In human Nrf2, the Neh2 domain (red) functions as the Keap1 binding and ubiquitinated region and the Neh1 domain (blue) functions as the DNA binding region. The OKD48 construct has 3xARE promoter (light blue), human Nrf2 (a.a.1–433; purple) and Flag-tagged luciferase (GL4; yellow). Under normal conditions, transcription of the OKD48 construct was not induced and the leaked OKD48 protein was degraded by the Keap1 (orange) system. Upon oxidative stress, OKD48 was transcriptionally induced by the 3xARE element and the resulting protein was stabilised by the Keap1 system. Thus, we detected luminescence only in cells experiencing oxidative stress.
To optimise the combination with stress-inducible promoters and the lengths of Nrf2 fragments, several types of construct were tested (Supplementary Fig. 1). As a result, the combination of three repeats of ARE promoter with Nrf2 fragment (a.a.1–433) showed the best response to the oxidative stressors ASN (sodium arsenite) and DEM (dimethylmaleate) (Supplementary Fig. 1). We named this construct OKD48 (Keap1-dependent Oxidative stress Detector, No-48) (Fig. 1) and used it for subsequent experiments.
Characterization of OKD48 in vitro
First, to test the functionality at the cellular level, OKD48 was transiently transfected to cells, treated with various agents and its activities were evaluated. Among various agents, OKD48 specifically and robustly responded to oxidative stressors (ASN or DEM) and barely responded to other stressors (ER stressors Tun (tunicamycin) or Tg (thapsigargin); reducing reagent DTT (dithiothreitol); apoptosis inducer via DNA damage from topoisomerase II inhibition Etp (etoposide); chemical hypoxia inducer TTFA (thenoyltrifluoroacetone)) (Fig. 2a). The specific response of OKD48 was also confirmed at the protein level: OKD48 protein was detected only with oxidative stressors, not with other agents (Fig. 2b). To check the response of the endogenous Keap1-Nrf2 pathway to these agents, the Nrf2 protein was examined by Western blot. As shown in Fig. 2c, the endogenous Nrf2 protein was significantly induced only by ASN or DEM but not by other regents, similarly to the case of OKD48. These results indicated that the response and specificity of OKD48 corresponded with the endogenous Keap1-Nrf2 pathway. Moreover, the examination of OKD48 with various concentrations of ASN (Fig. 3a) revealed its higher sensitivity than the traditional promoter-driven reporter (5x ARE in Fig. 3b). The activation of the endogenous Keap1-Nrf2 pathway under these conditions was evaluated by the induction of the HO-1 (Heme Oxygenase 1) mRNA, which is the transcriptional target of Nrf2 (Fig. 3c). These results imply the usefulness of OKD48 as an oxidative stress indicator in vitro.
Characterization of OKD48 in vitro.
(a) Specific response of the OKD48 to oxidative stress. The OKD48 construct was transfected into HeLa cells and luciferase assays were performed after treatment with or without various stresses (sodium arsenite (ASN), diethylmaleate (DEM), H2O2, tunicamycin (Tun), thapsigargin (Tg), dithiothreitol (DTT), etoposide (Etp) and thenoyltrifluoroacetone (TTFA)), for 8 hr or 16 hr. (b) Protein expression of OKD48 construct under various stresses. The OKD48 construct was transfected into HEK293T cells and then treated with or without various stresses for 8 hr. Their lysates were subjected to anti-Luc, anti-Keap1 or anti-GAPDH Western blotting. (c) Protein expression of endogenous Nrf2 under various stresses. HeLa cells were treated with or without various stresses for 8 hr or 16 hr, then their lysates were subjected to anti-Nrf2, anti-Keap1 or anti-GAPDH Western blotting.
Dose-dependent activation of OKD48.
(a, b) Luciferase assay with various concentrations of ASN. The OKD48 construct (a) and a promoter-driven reporter (b) were transfected into HeLa cells and luciferase assays were performed after treatment with or without various concentrations of ASN for 8 hr. (c) Induction of HO-1 with various concentrations of ASN. Total RNAs from each ASN-treated cells were subjected to quantitative PCR analysis. GAPDH was used as an internal standard.
Because OKD48 is regulated by the endogenous Keap1-Nrf2 pathway, there was a possibility that OKD48 produced a dominant negative effect on the cells. To eliminate this doubt, we evaluated the induction of the HO-1 (Heme Oxygenase 1) mRNA. As shown in Supplementary Fig. 2, HO-1 mRNA was induced at comparable levels both with and without OKD48, indicating that OKD48 does not have a dominant negative effect on the endogenous Keap1-Nrf2 pathway.
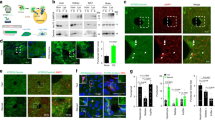
Next, the effects of Keap1 or Nrf2 for OKD48 were examined in vitro. As expected, the activity of OKD48 was greatly strengthened by overexpression with Nrf2 and also slightly activated by the treatments with ASN or DEM (Fig. 4a; left). The powerful activation by overexpression with Nrf2 was also confirmed at the protein level (Fig. 4a; right). On the contrary, overexpression with Keap1 attenuated OKD48 both with and without oxidative stress, though the stress response was maintained (Fig. 4b; left: cellular assay, right: Western blot). The effect of MG132, a proteasome inhibitor, was also examined. As shown in Fig. 4c, MG132 activated OKD48 more strongly than the oxidative stressors ASN or DEM. The stronger activation by MG132 was also found in endogenous Nrf2 (Fig. 4d). These results support the predicted working model that OKD48 is induced at the transcriptional level by Nrf2 and is degraded/stabilised at the post-translational level by Keap1.
Keap1-Nrf2-dependent regulation of OKD48.
(a) Effect of overexpression with Nrf2 on OKD48. Left: The OKD48 construct and Nrf2 overexpression vector were transfected into HeLa cells and luciferase assays were performed after treatment with or without sodium arsenite (ASN) and diethylmaleate (DEM) for 8 hr. Right: The OKD48 construct and Nrf2 overexpression vector were transfected into HEK293T cells and then treated with or without sodium arsenite (ASN) and diethylmaleate (DEM) for 8 hr. Their lysates were subjected to anti-Luc, anti-Keap1 or anti-GAPDH Western blotting. (b) Effect of overexpression with Keap1 on OKD48. Assays similar to those in Fig. 4a were performed with the Keap1 overexpression vector. (c) Effect of MG132 on OKD48. The OKD48 construct was transfected into HeLa cells and luciferase assays were performed after treatment with or without sodium arsenite (ASN), diethylmaleate (DEM) and MG132 for 8 hr. (d) Effect of MG132 on endogenous Nrf2 protein. HeLa cells were treated with or without sodium arsenite (ASN), diethylmaleate (DEM) and MG132 for 8 hr, then their lysates were subjected to anti-Nrf2, anti-Keap1 or anti-GAPDH Western blotting.
In addition to the luciferase-fused type, we also developed a GFP-fused indicator (Supplementary Fig. 3). Similarly to the luciferase version, the GFP-fused indicator (OKD48-venus) yielded significant fluorescence upon treatment with oxidative stressors (Supplementary Fig. 3a: fluorescence image; Supplementary Fig. 3b: fluorescence intensity with lysates). The specific induction of the GFP-fused indicator was also confirmed at the protein level (Supplementary Fig. 3c). These results suggest that the GFP-fused reporter (OKD48-venus) also functions as an oxidative stress indicator.
Generation of OKD48 transgenic mice
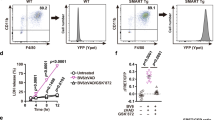
Given the functionality in vitro, we generated OKD48 transgenic mice and performed the following experiments. As expected, the resulting transgenic mice expressed the OKD48 transgene in all the organs that we examined (Fig. 5a). In the in vivo experiment, oxidative stress was induced by the intraperitoneal injection of ASN (Fig. 5) or DEM (Supplementary Fig. 4).
Generation of OKD48 transgenic mice.
(a) RT-PCR analysis of OKD48 transgene expression in various tissues. β-actin was used as an internal standard. (b) Luminescence activity in whole body. Mice were intraperitoneally injected with ASN. After 6 hr, the mice were analysed using the in vivo imaging system. (c, d) Luminescence activity in internal organs. The abdominally operated mice from Fig. 5b were analysed using the in vivo imaging system with wide range (c) or narrow range (d). (e) Luminescence activity in surgically-eviscerated organs. Liver, stomach, kidney and lung in Fig. 5b were surgically eviscerated and analysed using the in vivo imaging system. (f) Induction of HO-1 mRNA in liver. Total RNAs from mouse liver were subjected to quantitative PCR analysis. GAPDH was used as an internal standard.
Whole body analysis indicated that the ASN injection elicited significant signals from the thorax, abdomen (and weakly, from the lower abdomen) in the transgenic mice. In contrast, the control transgenic mice that received an injection of PBS showed very little luminescence, comparable with that of wild type mice (Fig. 5b). The signals were further precisely analysed by abdominal operation. The ASN-injected mice showed intense signals in almost all organs (Fig. 5c) and the liver elicited a particularly strong signal (Fig. 5d). Subsequent ex vivo analysis with surgically-eviscerated organs confirmed the signals from the liver, stomach, kidney and lung (Fig. 5e). Furthermore, the expressions of HO-1 mRNA in the liver were examined (Fig. 5f) and comparison analysis with these data indicated correlated activation between the OKD48 transgene and the endogenous Keap1-Nrf2 pathway. Also, as HO-1 was induced both in wild type mice and transgenic mice at comparable levels, the OKD48 transgene did not have a dominant negative effect on the endogenous Keap1-Nrf2 pathway.
Similarly to the case of ASN injection, luminescence signals were detected in the DEM-injected transgenic mice. Analysis by abdominal operation showed specific signals from the stomach and kidney in the DEM-injected transgenic mice, but not in the wild type mice or control mice (Supplementary Fig. 4a). Subsequent ex vivo analysis confirmed significant signals from surgically-eviscerated stomach (Supplementary Fig. 4b). Moreover, the examination of HO-1 mRNA confirmed a significant correlation between the luminescence signals and the activation of the endogenous Keap1-Nrf2 pathway in the kidney of DEM-injected mice (Supplementary Fig. 4c).
Characterization of OKD48 transgenic mice
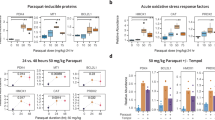
To test the in vivo functionality, we next performed three oxidative stress assays with OKD48 transgenic mice. First, we elucidated the signal intensity with several concentrations of ASN. As shown in Fig. 6a and b, OKD48 transgenic mice showed intense signals with the injection of 9.4 mg/kg ASN and the signals were further increased with 12.5 mg/kg ASN. However, no significant signal was detected with 6.25 mg/kg ASN or PBS. The examination of HO-1 mRNA confirmed a significant correlation between the luminescence signals and the activation of the endogenous oxidative stress response (Fig. 6c).
In vivo oxidative stress assay with OKD48 transgenic mice.
(a) Luminescence activity with several concentrations of ASN in internal organs. OKD48 transgenic mice were intraperitoneally injected with several concentrations of ASN. After 6 hr, the mice were abdominally operated and analysed using the in vivo imaging system. (b) Luminescence activity with several concentrations of ASN in liver. Livers in Fig. 6a were surgically eviscerated and analysed using the in vivo imaging system. (c) Induction of HO-1 mRNA with several concentrations in liver. Total RNAs from each ASN-injected mouse liver were subjected to quantitative PCR analysis. GAPDH was used as an internal standard. (d) Multiple detection of OKD48 signal in vivo. At day 1, OKD48 transgenic mice were intraperitoneally injected with ASN. After 6 hr, the mice were analysed using the in vivo imaging system. After an interval of 4 days (at day 5), the same individuals were re-injected with ASN and analysed similarly to day 1. (e) Luminescence activity with UV-A irradiation in vivo. Before (left) and after (right) UV-A irradiation (30 mW per cm2 (upper) and 5 mW per cm2 (lower)), OKD48 transgenic mice were analysed using the in vivo imaging system. (f) Induction of HO-1 mRNA with UV-A irradiation. Total RNAs from mouse skins irradiated with or without UV-A were subjected to quantitative PCR analysis. GAPDH was used as an internal standard.
Next, we performed multiple-round assays with OKD48 transgenic mice, taking advantage of the less-invasive method of in vivo imaging. As shown in Fig. 6d, the same individuals (upper, middle, lower for each) were sequentially injected with PBS or ASN at an interval of 4 days. The upper and middle were the control experiment confirming that the one-shot injection with ASN elicited significant signals at day 1 and day 5, respectively. Importantly, multiple injections at day 1 and day 5 both elicited intense signals repeatedly (Fig. 6d; lower). This indicated that the oxidative stress signal in OKD48 transgenic mice could be analysed repeatedly with the course of time.
Finally, OKD48 transgenic mice were subjected to UV-A irradiation, a milder and more physiological oxidative stimulus19 than toxically induced stress. As shown in Fig. 6e, the strong signal was detected with intense irradiation (30 mW per cm2; upper) and a significant signal was also detected even under more moderate condition with weak irradiation (5 mW per cm2; lower). However, no signal was detected before UV-A irradiation. The examination of HO-1 mRNA confirmed activation of the endogenous oxidative stress response with UV-A irradiation (Fig. 6f).
Thus, OKD48 transgenic mice could produce intense signals in line with the degree of stress damage, could be used for multiple-round assays and could detect both toxically induced and UV-A induced oxidative stress. The OKD48 transgenic mice would thus be useful for identifying organs or cells under oxidative stress in vivo.
Discussion
As described in the introduction, many recent studies have reported a significant connection between oxidative stress and various diseases and so an in vivo imaging system for oxidative stress has been awaited. Although a reporter mouse for oxidative stress has been previously reported20, its performance was not sufficient to detect the stress signal in vivo. In this study, we developed an oxidative stress indicator that is dually regulated by induction at the transcriptional level and by protein stabilisation at the post-translational level in the Keap1-Nrf2 pathway (Fig. 1). Our in vitro experiments showed that OKD48 works as a specific and sensitive indicator for oxidative stress, in a Keap1-Nrf2-dependent manner (Fig. 2–4). Also, experiments in transgenic animals showed that OKD48 functions as an indicator for oxidative stress in vivo (Fig. 5 and 6). This is the first system which can monitor oxidative stress with high sensitivity both in vitro and in vivo.
Previously, the activation of the Keap1-Nrf2 pathway has been monitored by the Western blot or Northern blot. These methods need cell lysis, involve multiple complicated procedures and are time-consuming. However, the newly-developed OKD48 system uses the luminescent reaction to overcome these problems and provides a simple, less-invasive and highly-sensitive detection method both in vitro and in vivo. This is a major advantage of our system.
The high S/N ratio of our system is achieved by combining the dual regulation mechanism from the Keap1-Nrf2 pathway. Previously, ARE-driven reporters have been used as a common tool for identifying transcription activators or inhibitors21. However, these reporters have an inherent drawback in terms of specificity. The consensus ARE sequence is TGAG/CNNNGC11, which overlaps with a 12-O-tetradecanoylphorbol 13-acetate (TPA) responsive element (TRE: TGAG/CTCA). In fact, many functional AREs found in target-gene regulatory regions include a TRE sequence22. Thus, the ARE-driven reporters are activated not only by Nrf2 but also by other transcription factors that bind to TRE23. To avoid this issue, we used a GSTYa fragment as the ARE promoter (GGAAATGACATTGCTAATGGTGACAAAGCAACTTT), which does not contain a typical TRE sequence. Furthermore, given the usefulness of the Nrf2-fusion23,24, we optimised the length of the fused Nrf2 fragment. The resulting fragment (a.a. 1–433 of Nrf2) contains a region other than the Neh2 domain (a.a. 1–93), which mainly contributes to the Keap1 binding and the stabilisation/degradation. This extra region (a.a. 94–433) might also contribute to the stress-dependent stabilization25 and thus to the high sensitivity of the OKD48 system. These elements in the OKD48 system at the transcriptional level and post-translational level contribute to minimise the side effects and maximise the sensitivity to oxidative stress.
OKD48 is a highly specific, sensitive system for screening Nrf2 inducers. There is a growing body of research suggesting that Nrf2-activating compounds will be potential therapeutic agents for various diseases such as cancers and neurodegeneration. In animal models, Nrf2-inducing compounds have a powerful ability to suppress chemical carcinogenesis by inducing both phase II detoxifying enzymes and antioxidant enzymes26. It has been reported that in an Alzheimer's disease model, Nrf2 activation improved symptoms27. To discover efficient Nrf2 inducers, high-throughput screening (HTS) of compound libraries will be one of the most powerful technologies24. We expect that HTS screening with the OKD48 system will allow highly efficient and specific screening of Nrf2 inducers. Moreover, the OKD48 transgenic mice also could be exploited to address various issues regarding oxidative stress in human diseases and drug development. By crossing the OKD48 transgenic mouse with a mouse model for a human disease, information about the status of oxidative stress during the course of disease could be obtained. As shown in Supplementary Fig. 5, intraperitoneally injected luciferin could reach the brain, though the efficiency was slightly less than that to the liver. Thus, OKD48 transgenic mice could also be useful for elucidating the oxidative status in a disease model relating to neurodegeneration. The OKD48 construct and the OKD48 transgenic mice could be powerful tools for addressing various issues regarding oxidative stress in human disease and drug development.
However, there are still some limitations to the usefulness of OKD48 transgenic mice. For example, weak oxidative stress associated with chronic diseases might not be detected in the mice. Indeed, we could not detect significant signals under physiological conditions, as detected in the case of our ER stress indicator28,29. One reason would be the low emission from the OKD48, though this indicator has an excellent S/N ratio. By fusing with the Nrf2 fragment, the luminescence ability of the luciferase portion might be partially inhibited. The use of other types of luminescence protein which have stronger light-emitting ability would overcome this problem; further research on this issue is required.
Nevertheless, OKD48 transgenic mice may provide valuable information regarding the oxidative status during development and under pathological conditions. Transient induction of oxidative stress in a limited portion of tissue may be a crucial trigger for disease progression. The OKD48 transgenic mice reported here will help clarify the mechanisms involved in oxidative stress and disease.
Methods
Plasmid construction
To make p(3xARE)TKbasal, a PCR-amplified ARE fragment (from mouse GSTYa promoter ACTAGTACTAGTGGAAATGACATTGCTAATGGTGACAAAGCAACTTTTCTAGA; attached restriction sites are underlined) was digested with SpeI-XbaI and self-ligated to form a triple-repeat fragment and further inserted into pTKbasal with XbaI-SpeI sites, which are located in the 5′ site of TK basal promoter30. The cDNA encoding human Nrf2 (a.a.1–433 in Fig. 1, or 1–93, 1–433, full-length in Supplementary Fig. 1) was PCR-amplified and inserted into the p(3xARE)TKbasal (or pTKX in Supplementary Fig. 1), with KpnI-XhoI sites. The cDNA encoding luciferase (GL4) was PCR-amplified with 1x Flag-tag on its 3′ terminal and inserted into p(3xARE)TKbasal-hNrf2(1–433) with XhoI-NheI sites. The resulting p(3xARE)TKbasal-hNrf2(1–433)-GL4-Flag was used as the OKD48 construct. We constructed its GFP version, p(3xARE)TKbasal-hNrf2(1–433)-Venus-Flag, in a similar way and used it as the OKD48-venus construct.
The overexpression vector of human Nrf2, pCAX-hNrf2, was constructed by inserting a PCR-amplified human Nrf2 fragment into pCAX with KpnI-XhoI sites. The overexpression vector of human Keap1, pCAX-hKeap1, was constructed by inserting a PCR-amplified human Keap1 fragment into pCAX with HindIII-XhoI sites.
Cell culture, transfection and treatment
HeLa cells and HEK293T cells were cultured at 37°C in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum, in an atmosphere containing 5% CO2. The calcium phosphate-DNA precipitation method was used to introduce plasmid DNA into the cells. To test cellular response to drugs, cells were treated with 10 μM (or various concentrations in Fig. 3) ASN (sodium arsenite), 100 μM DEM (diethylmaleate), 200 μM H2O2, 2.5 μg/ml tunicamycin, 1 μM thapsigargin, 1 mM DTT, 100 μM etoposide, 100 μg/ml TTFA (thenoyltrifluoroacetone), or 20 μM MG132 for the indicated time.
Luciferase assay
Dual luciferase assay was performed using the dual luciferase assay system (Promega) and a luminometer (Berthold). As an internal control, phRL-TK (Promega) was used. The results are shown as mean ± s.e.m. from triplicate experiments. Each value is shown as a fold induction normalised to that of each non-treated (for Fig. 2a, 3a and b), non-treated without Nrf2 overexpression (for Fig. 4a), or non-treated without Keap1 overexpression (for Fig. 4b), the value of which was set at 1.0. As a promoter-driven oxidative stress reporter, Cignal Reporter Assay Kit ARE (QIAGEN) was used in Fig. 3b.
Western blot analysis
Cells were lysed in SDS sample buffer (50 mM Tris-HCl (pH 6.8), 2% SDS, 50 mM DTT, 10% glycerol and 1 μg/ml bromophenol blue). The lysate was heated to 98°C for 10 min and SDS-PAGE was used to resolve the proteins in the lysate. After electrophoresis, the proteins were electrotransferred onto a polyvinylidene fluoride microporous membrane and immunodetected with a monoclonal antibody to luciferase (Promega), monoclonal antibody to GFP (Nacalai Tesque), monoclonal antibody to GAPDH (Cell Signaling Technology), monoclonal antibody to Keap1 (Cell Signaling Technology), or polyclonal antibody to Nrf2 (Santa Cruz) using standard procedures. The lysates from mice tissues were analysed similarly.
Quantitative PCR analysis
Quantitative PCR analysis of each transcript was performed using a TaqMan probe and 7900HT (Applied Biosystems) in Fig. 5f, Supplementary Fig. 2b, Supplementary Fig. 4c, or StepOneplus (Applied Biosystems) in Fig. 3c, 6c and f, in accordance with the manufacturer's instructions with GAPDH transcript as an internal control. Results are expressed as mean ± s.e.m. from triplicate experiments using RNA isolated from three independent samples. The probe/primer sets Hs01110250_m1 and Mm00516005_m1 were used for the quantification of human HO-1 and mouse HO-1 transcript, respectively.
Transgenic mice
The 4.5-kb SpeI-SfiI fragment of p(3xARE)TKbasal-hNrf2(1–433)-GL4-Flag was microinjected as a transgene into fertilised C57BL/6 mouse eggs and the transgenic offspring were screened by PCR using the primers 5′-ATC ACC AGA ACA CTC AGT GG-3′ and 5′-ACT CGG CGT AGG TAA TGT CC-3′. The resulting mice were used for in vivo imaging analysis. To induce oxidative stress for mice, ASN (12.5 mg/kg31 and various concentrations in Fig. 6a and b) or DEM (5 mmol/kg)32 was intraperitoneally injected. As a control for ASN, PBS was injected21. As a control for DEM, corn oil was injected32. UV irradiation was performed with a Blak-Ray High Intensity Lamp (100W, B-100A, 115V (UVP)) which emitted radiation of wavelength above 320 nM, providing 5 mW or 30 mW per cm2 UV-A. After 6–8 hr, the mice were intraperitoneally injected with D-luciferin (0.15 mg/g body weight) in PBS and analysed using the in vivo imaging system IVIS (Xenogen) according to standard protocols. Liver, kidney, stomach and lung were collected surgically from mice 10 minutes after luciferin injection, immersed in 300 μg/ml D-luciferin and analysed. All experimental protocols involving animals were approved by the Animal Studies Committees at RIKEN and Gunma University.
RT-PCR
In Fig. 5a, total RNA was prepared from mouse tissues using the Isogen reagent (Nippon Gene). A SuperScript first-strand synthesis system (Invitrogen) was used to synthesise the cDNA, according to the manufacturer's instructions. The target cDNA was amplified by 35 cycles of PCR using the following primers: GL4 sense primer, 5′-GTG GTG TGC AGC GAG AAT AG-3′; GL4 antisense primer, 5′-CCT CCT CGA AGC GGT ACA TG-3′; β-actin sense primer, 5′-ATG GAT GAC GAT ATC GCT-3′; and β-actin antisense primer, 5′-ATG AGG TAG TCT GTC AGG T-3′.
References
Sies, H. Oxidative stress: from basic research to clinical application. Am. J. Med. 91(3C), 31S–38S (1991).
Itoh, K. et al. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 15, 4184–4193 (1995).
Moi, P. et al. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 91, 9926–9930 (1996).
Itoh, K. et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 (1997).
Itoh, K. et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86 (1999).
Zhang, D. D. & Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 23, 8137–8151 (2003).
Cullinan, S. B. et al. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24, 8477–8486 (2004).
Kobayashi, A. et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24, 7130–7139 (2004).
Motohashi, H., Katsuoka, F., Engel, J. D. & Yamamoto, M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. USA. 101, 6379–6384 (2004).
Rushmore, T. H. & Pickett, C. B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 265, 14648–14653 (1990).
Rushmore, T. H., Morton, M. R. & Pickett, C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266, 11632–11639 (1991).
Wasserman, W. W. & Fahl, W. E. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA. 94, 5361–5366 (1997).
Spector, A. Oxidative stress and disease. J. Ocul. Pharmacol. Ther. 16, 193–201 (2000).
Johnson, J. A. et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann. NY Acad. Sci. 1147, 61–69 (2008).
Yoh, K. et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 60, 1343–1353 (2001).
Yamamoto, T. et al. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem. Biophys. Res. Commun. 321, 72–79 (2004).
Ishii, Y. et al. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 175, 6968–6975 (2005).
Motohashi, H. & Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557 (2004).
Allanson, M. & Reeve, V. E. Immunoprotective UVA (320–400 nm) irradiation upregulates heme oxygenase-1 in the dermis and epidermis of hairless mouse skin. J. Invest. Dermatol. 122, 1030–1036 (2004).
Johnson, D. A., Andrews, G. K., Xu, W. & Johnson, J. A. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J. Neurochem. 81, 1233–1241 (2002).
Igarashi, K. et al. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 367, 568–572 (1994).
Yamamoto, T. et al. Predictive base substitution rules that determine the binding and transcriptional specificity of Maf recognition elements. Genes Cells. 11, 575–591 (2006).
Hirotsu, Y., Katsuoka, F., Itoh, K. & Yamamoto, M. Nrf2 degron-fused reporter system: a new tool for specific evaluation of Nrf2 inducers. Genes Cells. In press (2011).
Smirnova, N. A. et al. Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem. Biol. 18, 752–765 (2011).
Sykiotis, G. P. & Bohmann, D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci. Signal. 3, 1–22 (2010).
Ramos-Gomez, M. et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA. 98, 3410–3415 (2001).
Kanninen, K. et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 106, 16505–16510 (2009).
Iwawaki, T., Akai, R., Kohno, K. & Miura, M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10, 98–102 (2004).
Iwawaki, T., Akai, R., Yamanaka, S. & Kohno, K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl. Acad. Sci. USA. 106, 16657–16662 (2009).
Oikawa, D., Kimata, Y., Kohno, K. & Iwawaki, T. Activation of mammalian IRE alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res. 315, 2496–2504 (2009).
Kimura, A. et al. Interferon-gamma plays protective roles in sodium arsenite-induced renal injury by up-regulating intrarenal multidrug resistance-associated protein 1 expression. Am. J. Pathol. 169, 1118–1128 (2006).
Goldring, C. E. et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 39, 1267–1276 (2004).
Acknowledgements
We are grateful for the support of BSI's Research Resources Center for DNA sequencing analysis. This work was supported by grants from RIKEN, JST, MEXT (No. 21790218, No. 22113524) to T. Iwawaki and from JSPS to D. Oikawa.
Author information
Authors and Affiliations
Contributions
D. Oikawa and T. Iwawaki wrote the main manuscript. R. Akai and M. Tokuda prepared figures 1–6. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplemental data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Oikawa, D., Akai, R., Tokuda, M. et al. A transgenic mouse model for monitoring oxidative stress. Sci Rep 2, 229 (2012). https://doi.org/10.1038/srep00229
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00229
This article is cited by
-
Induction of the antioxidant defense system using long-chain carotenoids extracted from extreme halophilic archaeon, Halovenus aranensis
International Microbiology (2022)
-
Nuclear transporter Importin-13 plays a key role in the oxidative stress transcriptional response
Nature Communications (2021)
-
Apelin/APJ signaling suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model
Scientific Reports (2020)
-
Kcnn2 blockade reverses learning deficits in a mouse model of fetal alcohol spectrum disorders
Nature Neuroscience (2020)
-
Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection
Molecular Biology Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.