Abstract
Antimicrobial peptides (AMPs), major innate immune effectors, are induced to protect hosts against invading microorganisms. AMPs are also induced under non-infectious stress; however, the signaling pathways of non-infectious stress-induced AMP expression are yet unclear. We demonstrated that growth-blocking peptide (GBP) is a potent cytokine that regulates stressor-induced AMP expression in insects. GBP overexpression in Drosophila elevated expression of AMPs. GBP-induced AMP expression did not require Toll and immune deficiency (Imd) pathway-related genes, but imd and basket were essential, indicating that GBP signaling in Drosophila did not use the orthodox Toll or Imd pathway but used the JNK pathway after association with the adaptor protein Imd. The enhancement of AMP expression by non-infectious physical or environmental stressors was apparent in controls but not in GBP-knockdown larvae. These results indicate that the Drosophila GBP signaling pathway mediates acute innate immune reactions under various stresses, regardless of whether they are infectious or non-infectious.
Similar content being viewed by others
Introduction
The innate immune system of animals provides the first and most primitive line of defense against invading microorganisms. Antimicrobial peptides (AMPs) are produced as immune effector molecules to fight pathogenic infection and the induction of AMPs is regulated through activation of the Toll and immune deficiency (Imd) pathways in Drosophila melanogaster1. Although the activation of both signaling pathways in response to infection has been extensively investigated, we also know that changes in innate immune activities are sometimes unrelated to microbial infection. Various physical and physiological factors such as temperature, starvation and diapause also elevate AMP expression levels2,3,4. It is also known that AMP expression is highly sensitive to developmental stage in mammals as well as insects5,6. Further, it has been recently reported that AMP expression in starved Drosophila is enhanced in response to the transcription factor FOXO, a key regulator of stress resistance, metabolism and ageing, independently of the immunoregulatory pathways7. Insulin signaling is currently the only known pathway for the induction of AMP expression by non-infectious stress. However, it is unlikely that animals cope with various non-infectious stressors by using the same signaling pathway that manages the regulation of innate immunity.
To investigate extracellular signaling in the innate immune regulation under non-infectious stresses, we focused on insect cytokines because cytokines in general regulate many physiological events including stress resistance through transmission of signals from outside the cell to the inside. While a large number of cytokines have been identified and their roles in mammals studied extensively, the number of known insect cytokines is quite limited. In Drosophila, spätzle is known as the cytokine that activates Toll signaling after microbial infection, which leads to expression of the target AMPs8. In lepidopteran insects, structurally similar bioactive peptides have been reported in the last 20 years and are now recognized as the insect cytokine family referred to as the ENF peptides on the basis of their common N-terminal sequence, Glu-Asn-Phe-9. These peptides are typically 23–25 amino acids long10,11,12,13,14,15,16,17 and growth-blocking peptide (GBP) was the first member of this peptide family discovered11. Other known insect-specific cytokines include Unpaired-318, which was first identified in Drosophila and hemocyte chemotactic peptide (HCP), identified in the armyworm Pseudaletia separata19. Stress-responsive peptide (SRP) has also recently been identified in the common cutworm Spodoptera litura20. Drosophila eda-like cell death trigger (eiger)21 and sex peptides22 have been reported to modify AMP expression levels. Among these insect cytokines, we focused on characterizing the functional role of GBP in innate immunity because GBP was initially identified as the factor responsible for the reduced growth exhibited by armyworm P. separata larvae under stress conditions such as parasitization by the parasitoid wasp Cotesia kariyai and exposure to low temperature23. NMR analysis of GBP showed that it consists of flexible N- and C-termini and a structured core stabilized by a disulfide bridge and a short antiparallel ß-sheet (ß-hairpin)24. Structural comparisons indicated that the core ß-hairpin region adopts the C-terminal subdomain structure of human epidermal growth factor. Consistent with this structural similarity, GBP at concentrations of 10−1–102 pmol/ml induced proliferation of human keratinocytes as well as insect Sf 9 cells25.
At least 16 members of the insect ENF cytokine family have been identified. They have diverse functions such as growth retardation11,12, paralysis induction13,15,16, cardioacceleration16, cell proliferation25,26, embryogenic morphogenesis27 and immune cell stimulation14,28. Characterization of some of these peptide cDNAs demonstrated that the ENF peptides are synthesized as a precursor form in which the active peptide is located at the C-terminal region15,28,29,30. Because it has been demonstrated that ENF family peptides stimulate insect immune cells like plasmatocytes to spread on foreign surfaces9,14,28, we first examined whether GBP affects humoral immune activity in a lepidopteran insect, the silkworm Bombyx mori. As expected, injection of B. mori GBP into B. mori larvae elevated the expression of AMPs. Further, GBP-induced elevation of AMP expression was demonstrated in silkworm larvae exposed to heat stress. Although this result demonstrated GBP-dependent induction of AMP expression in non-infected stressed silkworm larvae, elucidating in detail the pathway of GBP signaling in the immune system required analysis in Drosophila because little is known about the signaling pathways that activate AMP gene expression in non-Drosophila insects like B. mori. However, none of the ENF family cytokines have been identified in insect orders outside the Lepidoptera, making it necessary to identify the Drosophila GBP homolog9. Database searches did not reveal any obvious homologs in the fly genome, which suggested either that the Diptera lack ENF genes or that members of this gene family might have diverged too much to be identified on a sequence level in Diptera. Therefore, we first purified a peptidergic factor with GBP-like activity from the bluebottle fly Lucilia cuprina. Using the sequence of the bluebottle fly GBP homolog for motif and FASTA searches, we identified five Drosophila melanogaster homologs, among which CG15917 was most similar to lepidopteran GBPs in terms of the primary structure of its ORF. Overexpression and RNAi knockdown of GBP in the Drosophila larvae indicated that GBP regulates the expression of AMPs through a novel pathway associated with Imd and JNK. The Drosophila GBP signaling pathway stimulated AMP expression in Drosophila larvae in response to external stressors, whether they were infectious or non-infectious.
Results
Innate immune activity of B. mori GBP
The B. mori GBP homolog (BmGBP) is referred to as the ‘Bm paralytic peptide’ because it was initially isolated following induction of paralysis17. First, to examine whether BmGBP is involved in the humoral immune response of the silkworm, the antibacterial activity of the serum (hemolymph devoid of hemocytes) of B. mori larvae injected with BmGBP was measured. The antibacterial activity of the serum was clearly increased by injection of BmGBP as well as by injection of Serratia marcescens (Fig. 1a). The transcriptional enhancement was observed in all the AMP genes, Cecropin, Attacin, and Gloverin, tested in the BmGBP-injected larvae. The elevation of Cecropin expression was the highest of all the AMPs tested here (Fig. 1b) and the Cecropin expression increased linearly for 3 h following injection with 100 pmol/larva BmGBP and then decreased slightly (Fig. 1c). To confirm the contribution of BmGBP to protection of the larvae from bacterial infection, we compared the survival rates after S. marcescens injection of silkworm larvae pre-injected with anti-BmGBP IgG and those treated with non-immunized IgG. The larvae pre-injected with anti-BmGBP IgG were significantly more susceptible to bacterial infection than control larvae treated with non-immunized IgG (Fig. 1d), suggesting that BmGBP plays a vital role in defending silkworm larvae against bacterial infection. Further, heat treatment clearly elevated Cecropin expression, but the heat-induced elevation of its gene expression was slightly but significantly repressed by pretreatment with anti-BmGBP IgG (Fig. 1e).
Innate immune activity following bacterial challenge or BmGBP injection in silkworm larvae.
(a) Antibacterial activities were measured in the hemolymph of silkworm larvae injected with bacteria or BmGBP by the agar well diffusion method. Hemolymph was collected from test last instar larvae 12 h after injection of 100 ng/larva Serratia marcescens (Sm) or indicated doses of BmGBP. The supernatant after removing hemocytes by centrifugation was heated at 60°C for 10 min and then applied to a LB plate containing E. coli. BSA (10 nM) was used as the Control. Data are given as means±S.D. for five separate measurements. Asterisks indicate significant differences from control (t-test; *P<0.05, **P<0.01). (b) The bacterial challenge and BmGBP injection elevated all tested AMP expression levels. Total RNA was prepared from the isolated integument attached to the fat body of test larvae 3 h after injection of S. marcescens or indicated doses of BmGBP and AMP expression was measured by real-time quantitative PCR. BSA (10 nM) was used as the Control. Other explanations are as in (a). (c) Effect of time after injection of 100 pmol/larva BmGBP on Cecropin expression. Data are given as means±S.D. for five separate measurements. Asterisks indicate significant differences from value at 0 h (t-test; *P<0.05, **P<0.01). (d) Survival rates of silkworm larvae preinjected with anti-BmGBP antibody after infection with S. marcescens. Silkworm larvae pretreated with non-immunized antibody were used as control animals. Data are given as means±S.D. for four separate measurements. A significant difference between control (○) and test (•) larva slopes was determined using ANOVA with the general linear model procedures of Minitab (release 14, Minitab Inc., USA): P = 0.01. (e) Heat stress-induced elevation of Cecropin expression. Each silkworm larva preinjected with indicated dose of anti-BmGBP antibody was heated at 37°C for 9 h and its Cecropin expression level was measured 4 h after transferring them from 37°C to 25°C. NT: control larvae without heat treatment. NI IgG: Non-immunized IgG. Other explanations are as in (a).
Bluebottle fly and Drosophila GBP
D. melanogaster is an ideal insect with which to study the innate humoral immune response induced by GBP-like cytokines because much is known about its immune system; however, no Drosophila homolog of GBP has been identified. Because sequence searches did not reveal obvious homologs, we speculated that members of this gene family might have diverged too far to be identified on a sequence level. Therefore, we first tried to purify a peptidergic factor with GBP-like activity from Drosophila larvae, but they proved too small to yield enough peptidergic factor from their hemolymph. Thus, we started with the bluebottle fly L. cuprina larvae and used sequences from it to find GBP-like molecules in Drosophila. We isolated a bluebottle fly cytokine that induced strong aggregation of hemocytes (Supplementary Fig. 1) and sequenced the responsible peptide. This peptide is only 19 amino acids and shares a partial sequence similarity with lepidopteran GBPs (Fig. 2 and Supplementary Fig. 2); in particular, two cysteine residues are similarly located. It showed GBP-like activities such as hemocyte aggregation activity and mitogenic activity on High Five cells, an insect cell line derived from Trichoplusia ni (Fig. 2b, c). 5′- and 3′-RACE reactions with degenerate primers prepared based on the peptide sequence revealed a 435-bp cDNA with a 333-bp ORF (Supplementary Fig. 2) that encodes a precursor protein whose size is similar to those of lepidopteran GBP precursors (proGBPs). Further, the overall structure is consistent with the fact that lepidopteran GBPs are synthesized as pre-pro-peptides and are generated by cleavage of the C-termini of the proGBPs. Based on the structural features together with its biological activities, we concluded that the isolated peptide is the bluebottle fly homolog of lepidopteran GBPs.
Characterization of GBP homologs identified from Lucilia and Drosophila larvae.
(a) Primary structure of GBP homolog purified from the larval hemolymph of the bluebottle fly Lucilia cuprina. (b) Hemocyte aggregation and spreading induced by synthetic Lucilia GBP (final concentration: 10 nM). (c) Lucilia GBP induced cell growth activities of insect High Five cells. Data are given as means±S.D. for five separate measurements. Asterisks indicate significant differences from each control value (t-test; *P<0.05, **P<0.01). (d) C-terminal peptide sequences of the ORFs of Drosophila genes encoding homologous peptides to Lucilia GBP and lepidopteran GBPs. Common amino acid residues (in more than 3 peptides) are emphasized by yellow bands and conserved cysteine residues are indicated by stars. (e) RT-PCR analysis using primer pairs specific to each Drosophila gene identified as Drosophila homologs of GBP. Note that all gene expression bands are visible in Drosophila adults, but CG12517 and CG14069 expression bands are not present in larvae. (f) RT-PCR analysis of CG15917 expression in various tissues of Drosophila larvae: midgut (MG), Malpighian tubule (MT), integument (IM), fat body (FB), central nervous system (CN) and hemocytes (HC).
A cysteine-based motif search together with a FASTA search of the D. melanogaster genome database for the bluebottle fly GBP identified five genes encoding homologous peptides in the C-terminal regions of the ORFs (Fig. 2d). To determine which genes might be expressed in Drosophila larvae, we performed RT-PCR with gene-specific primer pairs using larval fat body mRNAs as templates and confirmed expression of three genes (CG11395, CG15917, and CG17244) among the five (Fig. 2e). RT-PCR using adult fat body mRNAs showed a striking contrast to the pattern of the larval gene expression: all five genes including CG12517 and CG14069 were expressed in adult fat body (Fig. 2e). To select the Drosophila GBP homolog most similar to lepidopteran GBPs, we applied two criteria: the ORF should consist of 100–200 amino acid residues and contain an arginine residue at an appropriate position (7–9 residues upstream of the first cysteine) for the preferential cleavage by a serine proteinase that is presumed to be a proGBP processing enzyme. Among the three candidate genes expressing in the larval fat body, only CG15917 fulfilled the criteria because it encoded a 118 amino acid protein that included an arginine residue (R94) located nine residues upstream of the first cysteine (Supplementary Fig. 3). RT-PCR with CG15917 gene-specific primers showed its expression in three organs: fat body, integuments and hemocytes (Fig. 2f). The expression level was the highest in fat body, very faint in hemocytes and the expression was undetectable in midgut and Malpighian tubules.
Characterization of Drosophila GBP (CG15917)
To confirm the structural and functional similarities of Drosophila GBP and lepidopteran GBP, we first examined whether Drosophila proGBP is processed as expected at the arginine residue (R94) in vivo. Western blotting of the extract prepared from control Drosophila larvae using the anti-Drosophila GBP antibody showed one main positive band of the predicted size (approximately 13 kDa) for proGBP together with one minor band that might be artificially derived from the main band protein. Exposure to temperature stress eliminated positive bands and produced one low molecular weight band of the predicted size (approximately 3 kDa) for GBP (Fig. 3A). N-terminal sequencing of the positive 3-kDa band peptide resulted in the predicted sequence of the active GBP, Ile-Leu-Leu-. In addition, a synthetic active GBP peptide induced aggregation of Drosophila y w larval hemocytes (Fig. 3b). Further, overexpression of CG15917 retarded larval development: pupariation of GBP-overexpressing larvae (hs-Gal4/UAS-proGBP or hs-Gal4/UAS-GBP) was delayed about 10–15 h as compared with control larvae (Fig. 3c, Supplementary Fig. 4). These results clearly demonstrated that the processing pattern of Drosophila proGBP was the same as that of lepidopteran proGBP and that Drosophila GBP also showed lepidopteran GBP-like activities, such as cellular immune function and larval growth retardation.
Characterization of structure and activities of Drosophila GBP.
(a) Western blot analysis of proGBP processing using anti-GBP IgG. Whole bodies of test Drosophila larvae placed at 4°C for 2 h were frozen immediately in liquid nitrogen 30 min after transferring them from 4°C to 25°C. Frozen bodies were homogenized as described in Materials and Methods and used for this analysis. Control larvae were constantly kept at 25°C. (b) Hemocytes isolated from Drosophila y w larvae were incubated with 100 nM GBP (or 100 nM BSA as the control) for 10 min. (c) Timing of pupariation of third instar larvae of transgenic Drosophila larvae. Same-staged larvae of control lines (hs-Gal4 and UAS-proGBP) and proGBP overexpression line (hs-Gal4/UAS-proGBP) were heated at 33°C for 45 min. (d) Elevation of GBP expression by S. marcescens injection (•) or by cold stress at 4°C (○). Data are given as means±S.D. for four separate measurements. Asterisks indicate significant differences from control (t-test; *P<0.05, **P<0.01).
GBP expression levels were examined in Drosophila larvae injected with S. marcescens or exposed to low temperature (4°C). The transcriptional enhancement was observed in both cases: GBP expression increased and reached a maximum level 4 h after S. marcescens injection while it reached a maximum 12 h after transfer from 25°C to 4°C (Fig. 3d).
Innate immune function of Drosophila GBP
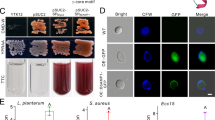
To determine whether the effects of Drosophila GBP on the Drosophila immune response were similar to those of BmGBP, we established transgenic lines carrying UAS-GBP and UAS-proGBP. Although overexpression of both Drosophila GBP and proGBP under the direction of an hs-Gal4 driver enhanced expression of most AMPs in the whole bodies of Drosophila larvae, the elevation was not as high as after bacterial infection (Fig. 4a). However, it was apparent that the AMP expression levels were much higher in the GBP-expressing larvae than in the proGBP-expressing larvae, indicating that the processing of proGBP to active GBP is prerequisite for the GBP-dependent elevation of AMP expression. Further, Metchnikowin (Mtk) expression showed the highest elevation of seven tested AMP genes. We then determined which tissue was the most sensitive to GBP in terms of elevation of Mtk expression by RT-PCR. Similar enhancements of Mtk expression were observed from 1 h after overexpression of GBP in all tissues tested: fat body, hemocytes and integument (Fig. 4b). To confirm the GBP-dependent enhancement of Mtk expression, transgenic larvae expressing green fluorescence protein (GFP) under the control of the Mtk promoter were used to visualize the spatial localization of the GBP-induced gene expression (Supplementary Fig. 5). Overexpression of GBP caused significant fluorescent signals in fat body. Further, injection of GBP peptide produced strong fluorescent signals in fat body and faint signals in epithelia, indicating that Mtk expression is activated by GBP in various tissues. Further, forced expression of GBP in adult males caused a significant elevation of Diptericin (Dpt) expression as well as Mtk expression. However, the elevation of Mtk expression was not as prominent as that in larvae with overexpression of GBP (Supplementary Fig. 6), implying the possibility that, although GBP elevates AMP expression in adults, there are some differences between adults and larvae in their immune responses to GBP.
Induction of AMP expression by GBP in Drosophila larvae.
(a) Induction of AMP expression by forced expression of proGBP or GBP under the direction of an hs-Gal4 driver in Drosophila larvae at 1 h (for GBP) or 3 h (for proGBP) after heat treatment or bacterial challenge (3 h after injection of heat-killed Serratia marcescens, OD600 = 1×10−2). The amount of mRNA was quantified by real-time quantitative PCR and rp49 was used to normalize the expression levels. Significant transcriptional enhancement of GBP (or proGBP) was observed in hs>UAS-GBP (or hs>UAS-proGBP) larvae within 30 min after heat treatment. Data are given as means±S.D. for four separate measurements. Asterisks indicate significant differences from control (t-test; *P<0.05, **P<0.01). (hs>UAS-GBP or hs>UAS-proGBP) hs-Gal4/UAS-GBP or hs-Gal4/UAS-proGBP. (b) Time course of Mtk expression after forced expression of GBP in fat body, hemocytes and integuments of Drosophila larvae. Other explanations are as in (a).
GBP enhances AMP gene expression in response to aseptic as well as septic wounding
To confirm the contribution of GBP to the regulation of Drosophila humoral immunity, Mtk and Dpt expression levels were examined in GBP RNAi knockdown larvae. Because we had verified a greater than 90% reduction of GBP expression in UAS-dsGBP;hs-Gal4 larvae without any accompanying morphological change in fat body 36 h after heat treatment (Supplementary Fig. 7), larvae of the RNAi knockdown line were subjected to a challenge with the Gram-negative bacterium S. marcescens. Although challenged control larvae exhibited robust expressions of Mtk and Dpt, targeting of GBP by RNAi significantly reduced the AMP expression (Fig. 5a). Measurement of the susceptibilities of GBP RNAi larvae to bacterial infection after injection of three different doses of S. marcescens showed that the GBP knockdown significantly increased sensitivity to the bacteria (Fig. 5b). Furthermore, counting the number of bacteria present in the hemolymph clearly demonstrated slower clearance in the GBP RNAi larvae compared with control larvae at 6 h after the challenge (Supplementary Fig. 8). Together, these data provide strong evidence that the GBP signaling pathway plays an essential role in the pathogen-induced innate immune response.
Disturbance of antimicrobial response in Drosophila GBP RNAi mutant larvae.
(a) Suppression of AMP expression in GBP RNAi larvae. AMP expression was measured at 0.5 h after Serratia marcescens challenge (48 h post-heat treatment, OD600 = 1×10−3) to transgenic larvae. Data are given as means±S.D. for four separate measurements. Asterisks indicate significant differences from controls (t-test; *P<0.01). (b) Survival rates of GBP RNAi larvae after bacterial challenge. Control and GBP RNAi larvae were stabbed with a very thin tungsten needle (diameter: approximately 0.05 mm) coated with indicated concentrations of Serratia marcescens, and the survival rates were measured at indicated times. Significant differences between control and test larval slopes were determined using ANOVA with the general linear model procedures of Minitab (release 14, Minitab Inc., USA): P = 0.001 for 10−3 and 5×10−3, P = 0.01 for 10−2. (c) AMP expression in control and GBP RNAi larvae after pinching them with forceps, preserving the barrier function of the overlying cuticle. Data are given as means±S.D. for five separate measurements. Asterisks indicate significant differences from one of the controls (results of hs-Gal4 larvae) (t-test; *P<0.05, **P<0.01). (d) AMP expression in control and GBP RNAi larvae 2 h after transferring them from 4°C to 25°C. Other explanations are as in (c). (e) AMP expression in control and GBP RNAi larvae after exposing them to 4°C. Other explanations are as in (c).
Because we found that BmGBP regulates noninfectious stress-induced elevation of Cecropin expression, we tested whether stressors associated with noninfectious stimuli induce GBP-dependent innate immune responses in Drosophila larvae. First, mortality rates after aseptic wounding were enhanced in GBP RNAi larvae compared to control larvae (Supplementary Fig. 9). The AMP expression patterns of the GBP RNAi and control larvae were compared after pinching them with forceps without breaching the barrier function of the overlying cuticle. When test larvae were pinched, Mtk and Dpt expression levels in the control larvae were much higher than in the GBP RNAi larvae, while no significant difference was observed between Drosomycin (Drs) expression levels in the control and GBP RNAi larvae (Fig. 5c). The patterns of stressor-dependent AMP expression in both control and GBP RNAi larvae were similarly reproduced by temperature change stress: Mtk and Dpt expression levels were elevated in control larvae but not in GBP RNAi larvae 2 h after transfer from 4°C to 25°C (Fig. 5d). Further, constant cold (4°C) stress-induced elevation of Mtk expression in larvae was also significantly repressed by GBP RNAi (Fig. 5e). These results strongly demonstrated that the GBP pathway quickly mediates AMP expression when larvae are physically or environmentally stimulated, regardless of infection. Therefore, it is supposed that the rapid induction of GBP-dependent AMP expression in Drosophila larvae contributes to protection from both infectious and non-infectious stressors.
GBP enhances AMP gene expression through IMD/JNK pathway
To characterize the GBP signaling pathway in the immune system of Drosophila larvae, the transcription levels of Mtk were analyzed in transgenic Drosophila larvae with disturbed expression of genes related to the innate immune system. Normal GBP-dependent enhancement of Mtk expression was observed despite loss-of-function disruption of the Toll pathway-related genes, spätzle, dorsal, and dorsal-related immune factor (Dif), indicating that GBP controls Mtk expression independently of the Toll pathway (Fig. 6a). By contrast, GBP did not induce Mtk expression in imd loss-of-function background larvae, but it did enhance Mtk expression in peptidoglycan recognition protein-LC (PGRP-LC), PGRP-LE and Relish (Rel) loss-of-function background larvae (Fig. 6a, b). We interpreted these results as indicating that Imd is essential for the GBP signaling pathway to enhance the AMP gene expression, but that its pathway is different from the orthodox Imd pathway.
GBP signaling pathway for AMP expression.
(a) Analysis of Mtk expression with various mutant backgrounds under the forced expression of GBP in Drosophila larvae. Induction of Mtk expression by overexpression of GBP under the direction of an hs-Gal4 driver in Drosophila larvae at 3 h after heat treatment. The J4 deletion represents a mutation of both Dif and dorsal. Data are given as means±S.D. for five separate measurements. Asterisks indicate significant differences from control (t-test; *P<0.05, **P<0.01). (b) Analysis of Mtk expression with PGRP-LC and PGRP-LE mutant backgrounds under forced expression of GBP in Drosophila larvae (hs-Gal4/UAS-GBP;LC and LE;hs-Gal4/UAS-GBP). Other explanations are as in (a). (c) Analysis of Mtk expression with bsk RNAi backgrounds under forced expression of GBP in Drosophila larvae. Induction of Mtk expression by forced expression of GBP was measured in larvae whose bsk expression was almost completely repressed as shown in Supplementary Fig. 10. Other explanations are as in (a). (d) In vitro analysis of Mtk expression with bsk knockdown backgrounds by Drosophila GBP peptide in fat body of Drosophila larvae. Fat body isolated from bsk knockdown larvae (actin-Gal4/UAS-dsbsk) was divided longitudinally into halves and each half was then incubated with 100 nM BSA or 100 nM GBP. The capacity of GBP to enhance Mtk expression was compared with its own control value (that in the other fat body half incubated with BSA). Other explanations are as in (a). (e) Model of AMP expression through the Drosophila GBP signaling pathway. GBP produced by processing of proGBP by various stressors may activate the GBP signaling pathway. Recruitment of adaptor protein Imd to the activated GBP receptor (?)-P77 adaptor protein complex may initiate the intracellular signaling to the induction of AMP expression via the JNK signaling. Although we have not yet isolated the GBP receptor, we have demonstrated its presence together with its adaptor protein (P77)26,34. The putative GBP receptor is indicated as ‘?’.
Immune activation of the Imd pathway has been reported to stimulate the JNK pathway due to a bifurcation at transforming growth factor-activated kinase 1 (TAK1)31,32. We therefore addressed whether JNK is involved in the GBP-dependent induction of Mtk expression. GBP-mediated induction of Mtk expression was not observed at all in basket (bsk) knockdown larvae (Fig. 6c, Supplementary Fig. 10), supporting our hypothesis that JNK signaling is required for proper activation of the GBP pathway for induction of Mtk expression. This interpretation was confirmed by an in vitro study indicating that the GBP peptide did not elevate Mtk expression in fat body isolated from bsk knockdown larvae, while the peptide clearly did elevate Mtk expression in fat body from control larvae (Fig. 6d). Further, we found that JNK was phosphorylated in the fat body of Drosophila larvae with overexpression of GBP. GBP-induced phosphorylation of JNK was also observed in Drosophila S2 cells 10 min after adding synthetic GBP to the culture medium (Supplementary Fig. 11).
Discussion
The present study revealed the innate immune activity of GBP in insects by demonstrating that it elevated expression levels of some AMPs in Drosophila larvae as well as in Bombyx larvae. In order to clarify the role and mechanism of GBP in inducing AMP expression in insects, we analyzed its signaling pathway in Drosophila because no detailed signaling pathway of an innate immune system had been recognized in non-Drosophila species. To perform the analyses, we first isolated a GBP-like cytokine from bluebottle fly larvae and then identified 46 Drosophila genes encoding its homologous peptide by a cysteine-based motif search (http://www.genome.jp/tools/motif/MOTIF2.html) using the C-(X except for C)6-8-C-(X except for C)1-4 pattern. Among these genes, CG14069 and CG17244 were selected as candidate Drosophila GBP genes whose ORFs consist of 100-200 amino acid residues. Subsequent FASTA searches (http://www.genome.jp/tools/fasta/) for the C-terminal 25-amino acid peptide sequence of CG14069 identified CG11395, CG15917 and CG12517. These five genes were found to encode GBP-like peptide sequences at the C-terminal ends. Among the five genes, we further selected the peptide gene most homologous to the bluebottle fly GBP and lepidopteran GBP genes based on the characteristics common to those GBP genes: the size (100–200 amino acids) of the ORF and the presence of an arginine residue at an appropriate position (7–9 residues upstream of the first cysteine). Further, after confirming the gene expression in the larval fat body, CG15917 remained as the most plausible candidate. The functional GBP peptide located in the C-terminal region of the CG15917 ORF stimulated aggregation of hemocytes from Drosophila y w larvae. Further, overexpression of CG15917 retarded larval development, creating a phenotype similar to that seen following injection of GBP into lepidopteran larvae10,11,12. We interpreted these results as an indication that the CG15917 product has a physiological role identical to that of lepidopteran GBPs.
Overexpression of proGBP as well as GBP significantly elevated Mtk and Dpt expression with the aid of the adaptor protein Imd in Drosophila larvae. The results are partially consistent with previously published data on the Drosophila gene CG15917: the gene was detected, along with many other genes, by microarray analysis as being transcriptionally activated three hours after septic injury of Drosophila adults33. We further revealed that the GBP-dependent induction of Mtk expression was abolished in larvae whose bsk expression was reduced by RNAi, indicating that the GBP pathway stimulated AMP expression through JNK signaling after recruitment of Imd to the activated GBP receptor (GBPR). This interpretation was partially confirmed by showing that GBP induced Mtk expression independently of PGRP-LC, PGRP-LE and Relish (Fig. 6a, b). The biological importance of GBP was demonstrated by the finding that RNAi targeting of GBP significantly repressed AMP expression during the initial phase of bacterial infection and consequently made the larvae more susceptible to the bacteria than control larvae. Moreover, GBP RNAi repression of AMP expression was found to occur when test larvae were subcutaneously damaged without bleeding by pinching. The GBP RNAi-induced repression of AMP expression was also observed in Drosophila larvae exposed to temperature stresses: AMP expression was significantly elevated in control larvae by transferring them from 4°C to 25°C or exposing to 4°C, but it was not seen in GBP RNAi larvae. The results clearly show that induction of GBP-dependent AMP expression does not require a pathogen-associated molecular pattern and that such non-infectious or non-injurious stimuli-dependent AMP expression is probably mediated by the GBP signaling pathway.
In lepidopteran larvae, GBP is abundantly present in a precursor form (proGBP) in hemolymph30,34. Our present experiments showed that this is also true for Drosophila. Non-injurious stimuli instantly triggered activation of GBP-processing enzyme(s) in hemolymph, by which the proGBP was proteolytically activated to GBP. The active GBP that resulted from the proteolytic processing under various stresses should trigger GBP-dependent AMP expression. It is reasonable to expect that such processing of proGBP enables the production of AMPs for the swift supply of active GBP under stress conditions. This interpretation is consistent with the fact that AMP expression was enhanced more by overexpression of active GBP than that of proGBP. Therefore, it is reasonable to propose that GBP serves as a key cytokine in the enhancement of AMP gene expression through the Imd/JNK pathway in Drosophila larvae exposed to various stressors, regardless of whether they are infectious or non-infectious (Fig. 6e).
Although there have been reports of the cross-regulation or cross-modification of two signaling pathways, Toll and Imd or JNK (or JAK/STAT) and NF-κB, our current knowledge is not still adequate to fully understand all mechanisms controlling the regulatory system35,36,37. Further, recent studies suggest the existence of an evolutionarily conserved mechanism of cross-regulation of metabolism and innate immunity7,38,39. It might be worth emphasizing that GBP was initially identified as a growth inhibitory factor in armyworm larvae. Although we confirmed that GBP overexpression retards normal larval development in Drosophila, we confirmed that this GBP-induced growth retardation did not affect AMP expression levels in test larvae under the present experimental conditions (Materials and Methods in Supplementary Information). Further, GBP-induced AMP expression was observed in Drosophila adults. Therefore, it is reasonable to propose that the insect cytokine GBP contributes to regulation of both growth and innate immunity. It may be possible to solve important problems concerning the adaptation of organismal defense to environmental stresses if we take the contributions of the novel signaling pathway through GBP-JNK into account there.
Methods
Animals
The silkworm Bombyx mori larvae were reared on an artificial diet at 25±1°C with a photoperiod of 16 h light:8 h dark. Larvae of the bluebottle fly, Lucilia cuprina, were reared on pork liver at 25±1°C and adults were fed dry milk, sugar and fresh water. Drosophila larvae and adults were reared on artificial food containing 8.7% (w/w) cornmeal, 5.2% (w/w) glucose, 3.5% (w/w) dried yeast, 0.3% antiseptic reagents and 0.8% (w/w) agar at 25±1°C. The transgenic strains UAS-GBP, UAS-proGBP and UAS-dsGBP were generated as described below and Mtk-GFP was gift from Jean-Luc Imler (Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France)40. UAS-dsbsk was supplied by NIG-FLY (National Institute of Genetics, Mishima, Japan). UAS-GFP, hs-Gal4, imd1, RelishE20, spätzlerm7, J4, PGRP-LE112 and PGRP-LC7454 were described elsewhere41,42.
Peptides and antibody
The Lucilia homologous peptide of GBP was purified by HPLC with reversed phase columns, C18, C4 and CN, as described in the Supplementary Information. Bombyx GBP (paralytic peptide)17, Lucilia GBP and Drosophila GBP (CG15917, 24-amino acid peptide in Supplementary Fig. 3) were synthesized by the solid phase method using standard Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry. Keyhole limpet hemocyanin-conjugated Drosophila GBP, emulsified in TiterMax Gold (TiterMax USA Inc.), was injected to immunize a rabbit. Anti-GBP IgG was prepared as described previously43.
Western blot analysis
Drosophila third instar larvae were frozen in liquid nitrogen immediately after temperature stress. Frozen bodies were quickly homogenized in 20 mM Tris-HCl (pH 7.0) containing 1% SDS and after centrifuging at 20,000 g for 10 min at 4°C the supernatant was separated by SDS/PAGE, then transferred onto an Immobilon-P PVDF membrane (Millipore). Western blot analysis with anti-Drosophila GBP IgG was carried out as described previously34.
Western blot analysis of JNK and phosphorylated JNK were carried out using the specific antibodies, anti-JNK (full length) (SC-) and anti-active JNK (V7931, Promega), according to the method of Igaki et al.44.
In vitro assay of GBPs in cultured cells and fat body
Insect High Five cells were purchased from Invitrogen and maintained in modified Grace's medium (MGM; Grace's medium containing 0.33% lactalbumin hydrolysate and 0.33% yeastolate) with 5% fetal bovine serum. After culturing the cells in MGM without fetal bovine serum or any growth factor at 25°C for 2 d, the cells were cultured in medium containing GBP plus 1 µCi of (3H)-thymidine for 32 h. The cells were washed three times with 0.15 M NaCl and lysed with 100 µl 0.3 N NaOH and the incorporated thymidine was counted using a liquid scintillation counter (Aloka LSC-5100)26. The cells were in their exponential growth phase at the time of labeling.
Third instar Drosophila larvae were cut longitudinally and the fat body was isolated. After washing with Grace's medium, the isolated fat body was separated into two almost equal halves and then one of two halves was incubated in Grace's insect medium containing BSA (control) and the other half incubated in the medium containing GBP. After incubation for indicated periods, total RNA was prepared from each fat body fragment as described previously42.
Establishment and analysis of transgenic fly
The GBP cDNA was obtained by PCR using fat body cDNA prepared from y w strain larvae. EcoRI and XbaI sites were introduced at the 5′ and 3′ ends, respectively, of the proGBP cDNA by using the following primer set: 5′-CGGAATTCAGTCATCGAAGCTTCATCGT-3′ and 5′-CGTCTAGAACACAAATATCACTTTATTGG-3′. Similarly, EcoRI and XbaI sites were introduced at the 5′ and 3′ ends, respectively, of the GBP cDNA by using the following primer set: 5′-AGAGTACAGAATTCATGATATTGCTGGAGACGACC-3′ and 5′-CGTCTAGAACACAAATATCACTTTATTGG-3′. Construction of the inverted repeat transgene of GBP was carried out according to the protocol of Lee and Carthe45 using the following primer sets: 5′-CGGAATTCATGTTGATACGTATTAATCC-3′, 5′-CGACTAGTTTACGCCGGCTTTCTGCATC-3′, 5′-CCACTAGTAGGTGAGTTTCTATTCGCGGT-3′, 5′-CCGCTAGCCTGAGTTTCAAATTGGTAAT-3′, 5′-CGGCTAGCTTACGCCGGCTTTCTGCATC-3′ and 5′-CGTCTAGAATGTTGATACGTATTAAATCC-3′. Each PCR product was subcloned into a pUAST vector. After checking the sequences of these constructs, they were injected into embryos. The Gal4-dependent overexpression or knockdown of GBP in Drosophila was induced by heat treatment at 35°C for 30 min.
Infection and aseptic wound experiments
Direct bacterial infection was induced by stabbing third instar larvae with a thin tungsten needle (diameter: approximately 0.05 mm) previously dipped into a concentrated culture of Serratia marcescens. The survival rates were measured at indicated times after the treatment.
The survival rates of third instar larvae aseptically injured with a thin sterilized tungsten needle (diameter: approximately 0.23 mm) were also measured by the same procedures as described above.
Sequence analysis
To identify Drosophila homologs of Lucilia GBP, we first performed a motif search for ‘C-(X except for C)6–8-C-(X except for C)1–4’ in the protein sequence database (KEGG GENES) on the MOTIF search web site (http://www.genome.jp/tools/motif/MOTIF2.html). Among 3,500 hit sequences, 46 were derived from Drosophila melanogaster genes and two candidate genes, CG11395 and CG14069, were selected by the criterion that the GBP-like sequence is located at the C-terminus of the ORF with 100–200 amino acid residues like lepidopteran GBPs. Subsequent FASTA analyses (http://www.genome.jp/tools/fasta/) using the C-terminal peptide sequence of CG14069 enabled us to identify CG12517, CG15917 and CG17244. Sequence similarities were also analyzed using the BLAST program against databases on the flybase (http://flybase.bio.indiana.edu/) web site.
For more details, see Supplementary Information (Materials and Methods).
References
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003).
Mitta, G. et al. Mytilin B & MGD2, two antimicrobial peptides of marine mussels: gene structure and expression analysis. Dev. Comp. Immunol. 24, 381–393 (2000).
Wu, J., Randle, K. E. & Wu, L. P. ird1 is a Vps15 homologue important for antibacterial immune responses in Drosophila. Cell. Microbiol. 9, 1073–1085 (2007).
Daybo, S., Kimura, M. T. & Goto, S. S. Upregulation of genes belonging to the drosomycin family in diapausing adults of Drosophila triauraria. Gene 278, 177–184 (2001).
Flatt, T. et al. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol. 211, 2712–2724 (2008).
Steinstraesser, L., Kraneburg, U., Jacobsen, F. & Al-Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunology 216, 322–333 (2011).
Becker, T. et al. FOXO-dependent regulation of innate immune homeostasis. Nature 463, 369–373 (2010).
Weber, A. N. et al. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat. Immunol. 4, 794–800 (2003).
Strand, M. R., Hayakawa, Y. & Clark, K. D. Plasmatocyte spreading peptide (PSP1) and growth blocking peptide (GBP) are multifunctional homologs. J. Insect Physiol. 46, 817–824 (2000).
Hayakawa, Y. Growth-blocking peptide: an insect biogenic peptide that prevents the onset of metamorphosis. J. Insect Physiol. 41, 1–6 (1995).
Hayakawa, Y. Juvenile hormone esterase activity repressive factor in the plasma of parasitized insect larvae. J. Biol. Chem. 265, 10813–10816 (1990).
Hayakawa, Y. Structure of a growth-blocking peptide present in parasitized insect hemolymph. J. Biol. Chem. 266, 7982–7984 (1991).
Skinner, W. S. et al. Isolation and identification of paralytic peptide from hemolymph of the lepidopteran insect Manduca sexta, Spodoptera exigua and Heliothis virescens. J. Biol. Chem. 266, 12873–12877 (1991).
Clark, K. D., Pech, L. L. & Strand, M. R. Isolation and identification of a plasmatocyte spreading peptide from hemolymph of the lepidopteran insect Pseudoplusia includens. J. Biol. Chem. 272, 23440–23447 (1997).
Wang, Y., Jiang, H. & Kanost, M. R. Biological activity of Manduca sexta paralytic and plasmatocyte spreading peptide and primary structure of its hemolymph precursor. Insect Biochem. Molec. Biol. 29, 1075–1086 (1999).
Furuya, K. et al. A cardioactive peptide from the southern armyworm Spodoptera eridanta. Peptides 20, 53–61 (1999).
Ha, S. D., Nagata, S., Suzuki, A. & Kataoka, H. Isolation and structure determination of a paralytic peptide from the hemolymph of the silkworm, Bombyx mori. Peptides 20, 561–568 (1999).
Agaisse, H. et al. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 5, 441–450 (2003).
Nakatogawa, S. et al. A novel peptide mediates aggregation and migration of hemocytes from an insect. Curr. Biol. 19, 779–785 (2009).
Yamaguchi, K., Matsumoto, H., Ochiai, M., Tsuzuki, S. & Hayakawa, Y. Enhanced expression of stress-responsive cytokine-like gene retards insect larval growth. Insect Biochem. Mol. Biol. doi: 10.1016/j.ibmb.2011.11.009 (2012).
Mabery, E. M. & Schneider, D. S. The Drosophila TNF ortholog eiger is required in the fat body for a robust immune response. J. Innate Immun. 2, 371–378 (2010).
Peng, J., Zipperlen, P. & Kubli, E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 15, 1690–1694 (2005).
Ohnishi, A. et al. Growth-blocking peptide titer during larval development of parasitized and cold-stressed armyworm. Insect Biochem. Molec. Biol. 25, 1121–1127 (1995).
Aizawa, T. et al. Solution structure of an insect growth factor, growth-blocking peptide. J. Biol. Chem. 274, 1887–1890 (1999).
Hayakawa, Y. & Ohnishi, A. Cell growth activity of growth–blocking peptide. Biochem. Biophys. Res. Commun. 250, 194–199 (1998).
Ohnishi, A., Oda, Y. & Hayakawa, Y. Characterization of receptors of insect cytokine, growth-blocking peptide, in human keratinocyte and insect Sf9 cells. J. Biol. Chem. 276, 37974–37979 (2001).
Tsuzuki, S. et al. A cytokine secreted from the suboesophageal body is essential for morphogenesis of the insect head. Mech. Dev. 122, 189–197 (2005).
Clark, K. D., Witherell, A. & Strand, M. R. Plasmatocyte spreading peptide is encoded by an mRNA differentially expressed in tissues of the moth Pseudoplusia includens. Biochem. Biophys. Res. Commun. 250, 479–485 (1999).
Hayakawa, Y. et al. Molecular cloning and characterization of cDNA for insect biogenic peptide, growth-blocking peptide. FEBS Lett. 376, 185–189 (1995).
Kamimura, M. et al. Molecular cloning of silkworm paralyrtic peptide and its developmental regulation. Biochem. Biophys. Res. Commun. 286, 67–73 (2001).
Silverman, N. et al. Immune activation of NF-kB and JNK requires Drosophila TAK1. J. Biol. Chem. 278, 48928–48934 (2003).
Park, J. et al. Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18, 584–594 (2004).
Boutros, M., Agaisse, H. & Perrimon, N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 3, 711–722 (2002).
Oda, Y. et al. Adaptor protein is essential for insect cytokine signaling in hemocytes. Proc. Natl Acad. Sci. USA 107, 158862–15867 (2010).
Tanji, T., Hu, X., Weberr, A. N. R. & Ip, Y. T. Toll and IMD pathways synergistically active an innate immune response in Drosophila melanogaster. Mol. Cell. Biol. 27, 4578–4588 (2006).
Delaney, J. R. et al. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 25, 3068–3077 (2006).
Kim, L. K. et al. Down-regulation of NF-kB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 5, e238 (2007).
Dionne, M. S., Pham, L. N., Shiras-Hiza, M. & Schneider, D. S. Akt and foxo dysregulation contribute to infection-induced wasting in Drosophila. .Curr. Biol. 16, 1977–1985 (2006).
DiAngelo, J. R., Bland, M. L., Bambina, S., Cherry, S. & Birnbaum, M. J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 106, 20853–20858 (2009).
Levashina, E. A., Ohresser, S., Lemaitre, B. & Imler, J. L. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide metchnikowin. J. Mol. Biol. 278, 515–527 (1998).
Takehana, A. et al. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 23, 4690–4700 (2004).
Lemaitre, B. et al. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Matsumoto, Y., Oda, Y., Uryu, M. & Hayakawa, Y. Insect cytokine growth-blocking peptide triggers a termination system of cellular immunity by inducing its binding protein. J. Biol. Chem. 278, 38579–38585 (2003).
Igaki, T. et al. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 21, 3009–3018 (2002).
Lee, Y. S. & Carthew, R. W. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30, 322–329 (2003).
Acknowledgements
We thank Jean-Luc Imler (Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France) for fly strains. This study was carried out under the Joint Research Program of the Institute of Low Temperature Science, Hokkaido University and supported by a grant-in-aid for Scientific Research (B) from the Japan Society for Promotion of Science (to YH).
Author information
Authors and Affiliations
Contributions
ST and MO carried out most experiments, HM and AO performed biochemical experiments and analysis, SK contributed to the development of transgenic flies by providing scientific inputs and suggestions and YH planned the study and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tsuzuki, S., Ochiai, M., Matsumoto, H. et al. Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Sci Rep 2, 210 (2012). https://doi.org/10.1038/srep00210
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00210
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.