Key Points
-
Highlights that planning and conducting a clinical trial is a lengthy process that must adhere to stringent regulations to ensure a high standard of ethics and reliability.
-
Explains that clinical trials are divided into multiple phases; regulatory approval must be obtained for each one.
-
Highlights that seeking approval is usually done after writing the protocol, securing funding and sponsorship, and appointing roles and responsibilities for members of the research team.
-
Highlights that in the UK, a unified application system is available to simplify the process of applying for regulatory approval.
Abstract
Seeking regulatory and ethical approval is a significant task that must be completed before conducting a clinical trial of a medical device. Currently in the UK, the Integrated Research Application System (IRAS) is a unified system for preparing regulatory, ethical and governance applications for the relevant bodies that must approve health and social research. This article outlines key aspects in planning a clinical trial of a medical device and how applications for approval can be prepared using IRAS.
Similar content being viewed by others
Introduction
Clinical trials explore the safety and efficacy of an intervention such as a medical device, medicinal product, procedure or strategy on human participants. Results are used to advance medical science and improve patient care. Planning and conducting a clinical trial is a lengthy process that must adhere to stringent regulations to ensure a high standard of ethics and reliability.1
Seeking regulatory and ethical approval is a significant task that must be completed before beginning research. Clinical trials are divided into multiple phases; regulatory approval must be obtained for each one. Seeking approval is usually done after writing the protocol, securing funding and sponsorship, and appointing roles and responsibilities for members of the research team. Currently in the UK, the Integrated Research Application System (IRAS) is the single unified system used to prepare regulatory and governance applications to relevant bodies for health and social research.
Medical devices are used every day in healthcare. Some examples specific to dentistry include dental instruments, dental prosthesis, orthodontic appliances and dental implants.
The aim of this article is to outline the process of planning a clinical trial of a medical device and provide guidance on how to complete the IRAS application. It is important to note that the regulatory and governance application processes change over time. Therefore, it is advisable to recheck with the relevant bodies regarding the up-to-date procedures.
Prior to planning
Before planning a clinical trial, it is important to have collected strong non-clinical data, finalised the product design and ensured a good manufacturing process of the device with quality assurance.
The Medicines and Healthcare products Regulatory Agency (MHRA) strongly advise giving advance notice as early as possible via the email devices.regulation@mhra.gov.uk, before submitting a formal application, stating some basic details about the investigational device, the intended population, the type of study, and estimated application date.2 Unlike clinical trials of medicinal products, registration of clinical trials of medical devices on the European Clinical Trials Database (EudraCT) is not required.3
Planning
Protocol
The protocol is the written document which states the research questions and how the clinical trial will be carried out to answer them. A typical document has headings for the introduction, project rationale, aims and objectives, study design and methodology, ethics, benefits, intercurrent and adverse events, resources and costs. A well thought out protocol is paramount to the success of the trial.4,5 The Health Research Authority (HRA) website offers resources and templates to support protocol write up.6
Funding
Commercial (for example, medical device manufacturers, pharmaceutical companies) and non-commercial (for example, government bodies, charities) sources can fund clinical trials.7 A comprehensive list of potential sources to seek funding from can be found on the HRA website.8 Local Research and Development (R&D) offices and the National Institute for Health Research Study Support Service9 can provide useful personalised advice on potential sources of funding and assist with projecting and attributing10 costs such as for equipment, IT, staff and data storage. NHS R&D Forum's website contains a list of NHS R&D offices.11 Regulatory bodies will check that adequate funding is available. Therefore, funding sources should be secured, and the protocol should be agreed by all stakeholders before submitting applications for regulatory approval.
Roles and responsibilities
Sponsorship
According to the UK Policy Framework for Health and Social Care research,12 all health research projects must have a sponsor. The sponsor is the individual, organisation or partnership that takes on overall responsibility for proportionate, effective arrangements being in place to set up, run and report a research project. There is potential for bias if the sponsor has considerable influence on the trial, and this is a common criticism of commercial trials.13,14
Chief investigator
The chief investigator (CI) is the lead researcher responsible for the overall conduct of a research project. CIs liaise with the Research Ethics Committee and necessary regulatory bodies during the project.12
Principal investigator(s)
The principal investigator(s) (PI) are the individuals responsible for carrying out the research at the research sites. If there is only one research site, this may be the same person as the CI. For multiple research sites, there will typically be one PI assigned to each site.
Capacity and capability
Capacity and capability to deliver the research should be assessed concurrent to the application process. This is the process by which the suitability of every potential participating trial site (NHS or Health Science Centre) is assessed based on patient populations, facilities, equipment and research staff. Site-specific information is required to set up projects within the NHS. The IRAS website explains the relevant routes in its guidance.15
Regulatory bodies
Under the Medical Devices Regulations 2002, ethical approval is essential before trialling medical devices on human participants. Clinical trials of medical devices require approval by the HRA in England, the Health and Care Research Wales (HCRW) in Wales or the local NHS/HSC R&D office in Northern Ireland or Scotland, a Research Ethics Committee and often the Medicines and Healthcare products Regulatory Agency (MHRA). Additional approvals are sometimes required such as when trials involve radioactive substances or for those involving human embryos or gametes.
Before a medical device is placed on the EU market, it must have a CE marking. This is the declaration by the manufacturer that the product complies with the essential requirements of the relevant legislation. For a medical device, CE marking indicates that the device has been approved by MHRA and conforms to EU directives relating to health, safety and environmental protection as approved by a UK notifiable body.16
The MHRA is the agency responsible for ensuring the efficacy and safety profiles of medicines, medical devices and blood components for transfusion in the UK.3 For medical devices, an MHRA application is needed for clinical trials funded commercially, or for clinical trials by a non-commercial organisation where commercialisation is intended. An MHRA application is not required when non-commercial organisations are not intending to commercialise a device, for example if a hospital department develops a novel device for its own use with no intention to collaborate with a commercial company. MHRA approval is also not required for trials of existing CE marked devices where the study involves the device's licenced indication.
The first few steps of the IRAS form will quickly establish and guide the user as to whether an MHRA application is required.
The HRA, which is now responsible for regulating health research, was established in 2011 to encourage and safeguard patients' and the public's interest in health research. The HRA manages the service responsible for the Research Ethics Committees UK-wide.
As of April 2018, 'HRA and HCRW approval' is the route by which any NHS research taking place in England or Wales applies for approval. It comprises a Research Ethics Committee review as well as undertaking the evaluation of governance and legal compliance. Therefore, this removes the need for each contributing organisation in England and Wales to perform local checks of compliance. HRA and HCRW approval is not used where research studies are led from Northern Ireland or Scotland. In these cases, local NHS/HSC management permission (R&D approval) is required as well as a separate NHS Research Ethics Committee review application.
Research Ethics Committees consider the ethical issues surrounding the proposed research. It is a requirement of the Research Governance Framework that opinions are provided within 60 days of submission, however, the average review times are significantly lower.17 Members of Research Ethics Committees include clinicians, academics and lay members. There are several opinions a Research Ethics Committee may give: favourable, favourable with conditions, provisional, unfavourable and no opinion. Studies given a favourable opinion with conditions or a provisional opinion require ongoing dialogue with the Research Ethics Committee in order to satisfy stated conditions, following which a favourable opinion may be granted.17 Ultimately, over 90% of studies are given a favourable opinion.
The Integrated Research Application System
IRAS is a single system allowing research applicants to seek approval from UK regulatory bodies in a simplified way. It saves time by avoiding duplication of entries into multiple application forms. Data gathered on unified forms within IRAS help generate populated application forms for relevant regulatory bodies including HRA, HCRW, MHRA, Human Fertilisation and Embryology Authority and others, therefore streamlining the application process.18,19 IRAS can be accessed at www.myresearchproject.org.uk, where an account must be created first with only basic registration details.20 Once registered and logged in, the 'Create a New Project' tab on the 'My Projects' page should be used to start the process of preparing a set of applications for a research project.
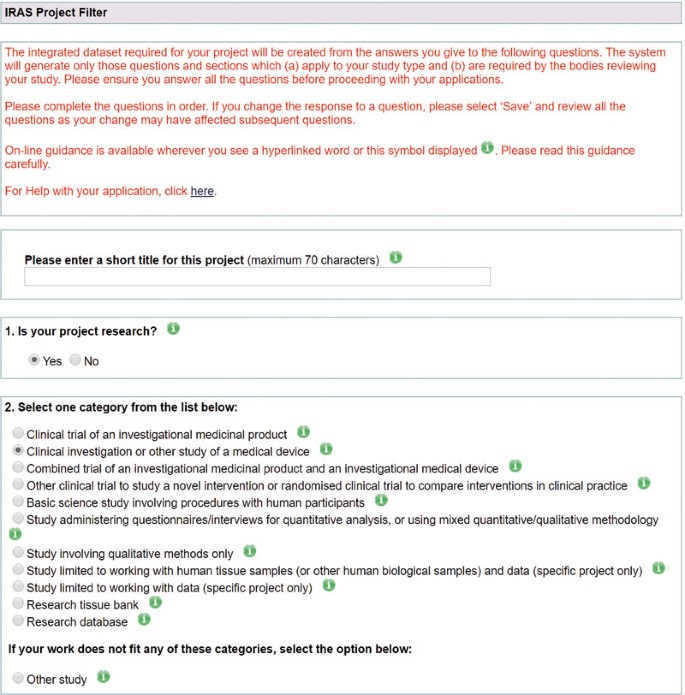
The process starts by filling a short form that asks about the type of research project in the 'IRAS Project Filter'. Completing this helps IRAS decide which application forms are needed for the research project and disables questions and sections that are not relevant. Figure 1 shows the first 2 questions of the project filter.
Reproduced from The Health Research Authority, The Integrated Research Application System (www.myresearchproject.org.uk)
As IRAS only prepares applications for research, the first question of the project filter asks whether this project is indeed research. If in doubt about whether a project is research, the HRA provides online decision tools21 and an enquiry line (hra.queries@nhs.net). The second question asks for the research category, one of the answer options being a clinical investigation or other study of a medical device. The page then asks specific questions including whether the trial is commercially sponsored, uses ionising radiation and involves human tissue samples. It also asks which country the research will be based, the location of the lead R&D office and if any NHS organisations are research sites.
After answering the questions and saving the form, the browser is redirected to the project navigation page where all the relevant forms are shown on the left – typically the IRAS form and possibly the MHRA form. The recommended way to start filling the forms is by using the 'Full Set of Project Data' or 'Integrated Dataset' which contains all the relevant sections and questions from all the forms that are required for this type of study.
The IRAS website has helpful guidance on making applications20 and each question within the form has question-specific guidance accessed by clicking the blue 'i' icon near the question.
MHRA application form
If the clinical trial requires an application to MHRA, the forms can be accessed via the 'MHRA Devices form' or the 'MHRA Medicines (EudraCT application) form' via IRAS. For trials of medicinal products which have received clinical trial authorisation, some of the form's dataset can be automatically imported from the EudraCT database. This feature is accessed by selecting the 'Import/Export' tab within the 'MHRA Medicines (EudraCT application) form'. However, this does not apply to medical devices.
IRAS Form Part A: Core study information
Section 1 (administrative details) asks for the full title of the project, details of the CI and correspondence information.
Section 2 (overview of the research) asks for a brief 300 word lay summary of the study and of the main significant ethical, legal or management issues and how they have been addressed. The summary is publishable on the HRA website if the research is reviewed by a Research Ethics Committee.22
Section 3 (purpose and design of the research) asks for the principle research questions/objectives, the secondary research questions/objectives, the scientific justification for the research, a summary of the design and methodology and the aspects of the research process where patients/service users/the public have been or will be involved. For clinical trials of medicinal products, the section will ask whether the product is licenced and what phase of the clinical trial this project represents (Phase I–IV).
Section 4 (risks and ethical issues, research procedures, recruitment and informed consent, confidentiality and publication and dissemination) asks for the principle inclusion and exclusion criteria, the clinical and non-clinical interventions/procedures for the participants and their potential risks, burdens and benefits. It then asks how the participants will be recruited for example, medical records or social media, how they will be first approached, how informed consent will be obtained if applicable, about storage and use of participant personal information and techniques to anonymise data, how data is analysed and by whom, participant incentives, reimbursements and payments, conflicts of interests with regards to sponsorship and funding, whether other health professionals will be informed, that is, the patient's GP, and the intended publication and dissemination of the research findings.
Section 5 (scientific and statistical review) asks about details of the statistician if applicable, as well as the primary and secondary outcome measures, the study sample size, participant allocation method and method of data analysis.
Finally, section 6 (management of the research) starts by asking for the details of the key collaborators, research sponsor(s), funder(s) and lead NHS R&D contact. It also asks for the expected length of time for the study in the UK and/or other countries and what defines the end of the trial, what criteria will cause premature termination of the study, whether the trial is single or multicentred, which country will host it, which organisation is responsible, how the research will be conducted and audited, indemnity, and whether the sponsor has arranged for payment compensation to participants that are harmed during the study.
IRAS Form Part B: Additional information
Part B of the application form is more specific to the type of study being carried out. This will have been determined by the initial project filter page.
For a clinical trial of a medical device, there will be further questions on classification, site of manufacture, number of devices in the trial, safety features, whether it is an active device or implanted/invasive device, sterilisation considerations and if animal studies have been undertaken.
Although IRAS contains questions relating to date of payment of fees to the MHRA, the payment process was changed in April 2017. Advance payment is no longer required, so proof of payment is not necessary when submitting the application. An invoice will be sent for the correct amount once the application has been validated by MHRA.2
IRAS Form Part C: List of research sites
Details of the host organisations responsible for the research sites are entered in this section.
IRAS Form Part D: Declarations
Declarations must be made by the CI, sponsor's representative and/or academic supervisor and/or information guardian, either by electronic authorisation or ink signature. Electronic authorisations are compulsory for applications to NHS Research Ethics Committees.
Supporting documents
The 'Checklist' tab in the IRAS form and the MHRA Medical Devices form show the required and optional supporting documents that need to be sent with the application. The answers provided in the project filter will help the system determine which documents are required. For a clinical trial of a medical device, the minimum required documents are the research protocol or proposal, a summary two-page curriculum vitae for the CI and a costing template. The MHRA application also requires a clinical investigator brochure which is a compilation of the clinical and non-clinical data on the device that are relevant to the study. If available, the instructions for use of the medical device should also be uploaded.
Submitting the application
In England, the IRAS form can be submitted electronically to HRA by following the instructions on the 'E-submission' tab. Any supporting documents that have been uploaded on the 'Checklist' tab will also be submitted. In Scotland and Northern Ireland, the IRAS form can be submitted electronically for Research Ethics Committee review and contains guidance on how to export and prepare files to be emailed to the local NHS/HSC R&D office.
For the MHRA application, PCA1 and PCA2 forms are generated by IRAS which need to be printed and signed. These forms, along with supporting documents need to be transferred onto CDs to be sent by post to MHRA. The most up-to-date postal address, guidance on how many CDs are required and labelling instructions are found at https://www.gov.uk/guidance/notify-mhra-about-a-clinical-investigation-for-a-medical-device.2
Conclusion
The aim of IRAS is to collate all necessary information and unify the process of preparing applications for regulatory approval. Research applications will continue to become more centralised. While seemingly daunting at first, users can quickly become familiar with the system and the systematic method of submission by navigating through the IRAS website whether by preparing the first submission or by creating a dummy research project.
References
Kerr D J, Knox K, Robertson D, Stewart D, Watson R . Clinical trials explained: A guide to clinical trials in the NHS for healthcare professionals. John Wiley & Sons, 2008.
Medicines and Healthcare products Regulatory Agency. Notify MHRA about a clinical investigation for a medical device. 2018. Available at https://www.gov.uk/guidance/notify-mhra-about-a-clinical-investigation-for-a-medical-device (accessed February 2018).
Abozguia K, Phan T T, Shivu G N, Maher A, Ahmed I, Frenneaux M P . Insights into how to conduct a clinical trial in the UK. J R Soc Med 2007; 100: 469–472.
Silverman R, Kwiatkowski T . Research fundamentals: III. elements of a research protocol for clinical trials. Acad Emerg Med 1998; 5: 1218–1223.
Chan A W, Tetzlaff J M, Altman D G et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013; 158: 200–207.
Health Research Authority. Protocol. 2017. Available at https://www.hra.nhs.uk/planning-and-improving-research/research-planning/protocol/ (accessed February 2018).
Ehrhardt S, Appel L J, Meinert C L . Trends in National Institutes of Health Funding for Clinical Trials Registered in ClinicalTrials.gov. JAMA 2015; 314: 2566–2567.
Health Research Authority. Research Funding. 2017. Available at https://www.hra.nhs.uk/planning-and-improving-research/research-planning/funding/ (accessed August 2017).
National Institute for Health Research. Study support services. Available at https://www.nihr.ac.uk/funding-and-support/study-support-service/ (accessed February 2018).
Department of Health and Social Care. Attributing the costs of health and social care Research & Development (AcoRD). 2012. Available at https://www.gov.uk/government/news/attributing-the-costs-of-health-social-care-research-development-acord (accessed February 2018).
NHS Research and Development Forum. National Directory of NHS Research Offices. Available at http://www.rdforum.nhs.uk/content/contact-details/ (accessed February 2018).
Health Research Authority, Department of Health (England), Ireland) DoHN, Scottish Government Health and Social Care Directorates, (Wales) DfHaSS. UK policy framework for health and social care research. 2018.
Kjaergard L L, Als-Nielsen B. Association between competing interests and authors' conclusions: epidemiological study of randomised clinical trials published in the BMJ. BMJ 2002; 325: 249.
Lexchin J, Bero L A, Djulbegovic B, Clark O . Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003; 326: 1167–1170.
Integrated Research Application System. Site specific information. 2017. Available at https://www.myresearchproject.org.uk/help/hlpsitespecific.aspx (accessed February 2018).
Department for Business Energy & Industrial Strategy. CE Marking. 2012. Available at https://www.gov.uk/guidance/ce-marking (accessed July 2018).
Health Research Authority. Research Ethics Committee – Standard Operating Procedures. 2016.
Smajdor A, Sydes M R, Gelling L, Wilkinson M . Applying for ethical approval for research in the United Kingdom. BMJ 2009; 339: b4013.
Health Research Authority. Integrated Research Application System (IRAS). 2017. Available at https://www.myresearchproject.org.uk/ (accessed February 2018).
Integrated Research Application System. Using IRAS. Available at https://www.myresearchproject.org.uk/Help/HelpPage.aspx (accessed February 2018).
Health Research Authority, Medical Research Council. Is my study research? Available at http://www.hra-decisiontools.org.uk/research/ (accessed February 2018).
Health Research Authority. Research Summaries. Available at https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/ (accessed February 2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ria, S., Bakir, A., Abeysiri, S. et al. Applying for regulatory approval of a clinical trial of a medical device in the UK – A practical guide. Br Dent J 225, 1033–1036 (2018). https://doi.org/10.1038/sj.bdj.2018.1033
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2018.1033