Abstract
Governments have attempted to increase clinical trial activity in their jurisdictions using a range of methods including simplifying the ethics review and governance process of clinical trials. This study’s objective was to systematically review the effects of government actions targeting ethics reviews or governance processes on clinical trial activity. The data sources of Pub Med, Scopus, Sage, ProQuest, Google, Google Scholar and reference lists were all searched between 9/8/20 and 6/9/20. From these sources, 1455 potentially eligible reports were reviewed and full text assessments were done for 295. Thirty-eight reports provided data on 45 interventions—13 targeting ethics review and 32 targeting governance processes—were included. There were data describing effects on a primary or secondary outcome (the number of clinical trials or expenditure on clinical trials) for 39/45 of the interventions. 23/39 (59%) reported positive effects, meaning a greater number of trials and/or expenditure on clinical trials (6/11 ethics, 17/28 governance), 7/39 (18%) reported null effects (4/11 ethics, 3/28 governance) and 9/39 (23%) reported adverse effects (1/13 ethics, 8/28 governance). Positive effects were attributable to interventions that better defined the scope of review, placed clear expectations on timelines or sought to achieve mutual acceptance of ethics review outcomes. Adverse effects were mostly caused by governance interventions that unintentionally added an extra layer of bureaucracy or were developed without full consideration of the broader clinical trial approval system. Governments have an opportunity to enhance clinical trial activity with interventions targeting ethics reviews and governance processes but must be aware that some interventions can have an adverse impact.
Similar content being viewed by others
Introduction
Randomised controlled clinical trials are gold standard research investigations designed to generate high-quality data about ways to prevent, detect or treat medical conditions (NHMRC National Health and Medical Research Council, Australian Clinical Trials (2021)). If done well, the evidence that derives from clinical trials forms the basis for the implementation of new health interventions, clinical guidelines and government policy. Clinical trials have also become important sources of employment and external investment for some jurisdictions (DOH Department of Health, 2021), as well as providing a means for the community to access novel therapies earlier.
The regulation and governance of clinical trials has evolved in a piecemeal fashion in most jurisdictions and the responsibilities of different parties are often poorly defined. Processes may be overlapping, bureaucratic and highly varied across clinical sites requiring reduplication of effort, enormous resources, and extended timelines. A 2013 Government of Australia review found that ‘Australia has become one of the most expensive locations for clinical trials in the world and is inefficient in ethics approvals and governance processes’ (McKeon et al., 2013). The effect of overlapping and bureaucratic approval processes for clinical trials can prevent researchers accessing new medicines for evaluation, reduce investment in the health sector and cost lives. In Australia, for example, regulatory delay is estimated to be the cause of up to 60 premature deaths each year in oncology patients because research is slowed and patient access to novel therapies is delayed (Whitney and Schneider, 2011). Similarly, a UK study found that delays in approving studies frequently stretched to over a year with extended and inefficient use of trial coordinator time being borne by studies (Hackshaw et al., 2008). And in Japan, Konishi et al. highlighted the example of a medical device that was required to have a Japanese trial arm added, resulting in 4 years’ delay of device approval compared with US timelines) (Konishi et al., 2018).
Ethics review and governance have been the target of multiple government interventions designed to increase clinical trial activity (Zhang et al., 2015; Kong, 2007; Madhani, 2010; Sarma and Manisha, 2018; Srinivasan, 2009). Ethics review describes the formal evaluation of the moral grounding of the proposed research project and governance the processes used by institutions to ensure that they are accountable for research conducted under their auspices. In general, interventions have attempted to simplify and harmonise ethics and governance systems and while some interventions have been successful (Konishi et al., 2018), others have not (Berge et al., 2015). The objective of this paper was to systematically collate and summarise evidence describing the effects of interventions that have sought to increase clinical trial activity by reforming ethics review or governance processes.
Methods
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2021). The guiding question was: ‘What are the effects of governments actions targeting ethics or governance processes on clinical trial activity?’ The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42020191510 as a slightly broader question of ‘What are the effects of governments actions on clinical trial activity?’. Other government actions such as tax credits or funding initiatives will be addressed in a separate publication, due to a large number of retrieved studies, which made reporting in one manuscript not feasible.
Search strategy
The search strategy was developed in consultation with the UNSW Library research service where key search terms were identified (‘clinical trials’ and ‘public policy’ as free text keywords). These terms were combined using the Boolean operator ‘AND’ to complete searches of Pub Med, Scopus, Sage, ProQuest and Google Scholar databases. This was followed by a search of the internet for grey literature done using the same terms in the search engine Google. Finally, a hand search of the references of all included reports was done. No time constraints or language barriers were placed on the search parameters.
The reports identified from the searches of Pub Med, Scopus, Sage and ProQuest were exported to Covidence, which automatically removed duplicate entries. The reports identified from Google Scholar were exported to Publish or Perish. The Google search engine results as well as the reports identified from the hand searches of reference lists were recorded in an Excel spreadsheet and duplicates were excluded by hand.
Study inclusion criteria
Studies were eligible for inclusion if they (1) reported on a policy intervention of interest (ethics review or governance process); (2) provided some report on the impact of the intervention; and (3) the intervention was implemented by a national or sub-national jurisdiction. Studies that analysed a jurisdiction’s clinical trial sector or the laws and regulations that contributed to ethics review and/or governance processes but did not report on the effects of a specific intervention were excluded. ‘Governance processes’ were taken to include all approvals necessary for a trial to be initiated at a site—except ethics evaluation processes. This might include, site contracts, regulatory submissions and site required initiations. Studies that identified the implementation of an eligible intervention but failed to report on an outcome of interest were recorded in the listings but noted to have missing outcome data.
Study selection
Two authors (SC and ER) independently screened all potentially eligible studies. For the studies identified from Pub Med, Scopus, Sage and ProQuest this comprised an initial review of titles and abstracts with review of the full text articles done only for those that passed initial screening. For the studies identified from Google Scholar and using the Google search engine the screening was a single step process. Where one reviewer included or excluded a study in contradiction to the second reviewer a discussion was had, and consensus was reached about whether the study was eligible.
Data extraction
Two authors (SC and ER) independently extracted data from each eligible study into separate copies of the same spreadsheet. Once both authors had completed the data extraction process every item of data was compared and discrepancies were reconciled by discussion. The study characteristics extracted were country, year of publication, intervention (ethics or governance), impact of each intervention on outcomes of interest (number of trials, expenditure on trials, other assessment of impact).
Quality assessment
As intervention studies, the quality of each was assessed by four parameters as advised by the Cochrane Handbook for Systematic Reviews (Higgins et al., 2021). The four parameters were confounding bias that arises when there are systematic differences between experimental intervention and comparator groups in the care provided, which represent a deviation from the intended interventions; selection bias that arises when later follow-up is missing for individuals initially included and followed, bias due to exclusion of individuals with missing information about intervention status or other variables such as confounders; information bias introduced by either differential or non-differential errors in measurement of outcome data; and reporting bias representing selective reporting of results from among multiple measurements of the outcome, analyses or subgroups in a way that depends on the findings.
Categorisation of interventions
The ethics review interventions were divided into the categories of: single application (Industry CDo, 2011; Thompson et al., 2009; Care ACoSaQiH, 2020; Warlow, 2005) (researchers doing multicentre trials need only make once central application that is binding on all research sites within that jurisdiction); mutual acceptance (Evans, Zalcberg (2016); Thompson et al., 2009; Care ACoSaQiH, 2020), (Ethics Committees accept approvals made by other Ethics Committees without requiring re-assessment); streamlined approval mechanisms (Thompson et al., 2009; Care ACoSaQiH, 2020) (such as mandated maximum response times); scope guidelines (Sarma, Manisha (2018); Nakamura et al., 2003) (that constrain Ethics Committees to address specific issues only); or other (Zannad et al., 2019; Kong, 2007).
The governance interventions were divided into: co-ordinating centre (Thompson et al., 2009; Care ACoSaQiH, 2020; Industry CDo, 2011; Committee UHoCSaT, 2013), (a new government office was implemented to shepherd trials through the approval pathway); scope guidelines (Madhani, 2010; Mani, 2006; Sarma, Manisha (2018)), (governance bodies were encouraged to process applications in a particular way); single application (Srinivasan et al., 2009; Hudson et al., 2016; Srinivasan, 2009; Haynes et al., 2010), (using a centralised governance body for all institutions); streamlined approval (Fudge et al., 2010; Ippoliti, Falavigna (2014); Choudhury, Saberwal (2019)), (whereby applications were given some form of special treatment or consideration for rapid approval), other regulatory changes (Kong, 2007; Mossialos et al., 2016; Zhang et al., 2015; Caulfield 2001; Thompson, 2014; van Oijen et al., 2017; Reith et al., 2013; Berge et al., 2015; McGee, 2006; Warlow, 2005; Care ACoSaQiH, 2020; Chen, 1998; Ikegami, Campbell (1999); Konishi et al., 2018; Hackshaw et al., 2008; Newman et al., 2016; Kwon, Jung (2018); ATIC Australian Trade and Investment Commission (2018); Chengodu, 2013; Webster, Temple-Smith (2013)), (a range of different changes to the regulatory process) or other (non-regulatory governance interventions, including programmes focused on knowledge sharing, safety, or specific programmes for orphan drugs). While some interventions included aspects of another, they were categorised according to the primary objective of the intervention strategy.
Outcomes
The primary outcome of interest was the number of clinical trials. Secondary outcomes were financial impact and community access to quality healthcare. Community access to quality healthcare was discontinued as an outcome since there was little reporting on this outcome. ‘Financial impact’ was measured by expenditure on clinical trials, which was defined as funding for trial activity from any source but most data related to expenditure on trials by multinational healthcare companies. For both the primary and secondary outcomes the effects were reported as positive, null or adverse.
Data synthesis
Outcome data about effects on the number of trials, expenditure on trials and other outcomes were described inconsistently and using different metrics across studies. To enable the effects of interventions on each outcome to be summarised, the effect of each intervention on each outcome was documented as positive (when a favourable impact was identified and the number of trials or expenditure on trials increased), null (when no impact on the number of trials or expenditure on trials was identified), adverse (when a negative effect on the number of trials or expenditure on trials was identified) or missing. The numbers of studies reporting each form of outcome was summarised and presented in tabular and graphical formats.
Results
Identified studies
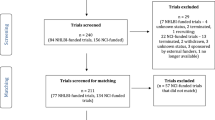
There were a total of 1455 potentially relevant reports identified in the database searches (Fig. 1). One-hundred fifty-four reports were retrieved from peer reviewed databases and examined in Covidence. 9820 were identified from Google Scholar and the first 980 (10%) were exported to publish or perish for title and abstract review. The first 100 titles reviewed yielded 20 studies for full text review with this number continually diminishing to only 3 studies in the last 80 reviewed (Supplementary Appendix 1). An additional 200 reports were identified from the Google search engine and were similarly reviewed and recorded in Excel. One-hundred seventy-four of these reports were identified as potentially relevant and their bibliographies were reviewed resulting in an additional 94 potentially relevant reports. The bibliographies of these 94 reports were then examined and a further 27 potentially relevant reports were identified for review. In total 295 reports were deemed relevant for full text review with 257 excluded as failing to meet the inclusion criteria. This left 38 reports with data describing 45 distinct interventions. 14 of these reports were published in the last 5 years, 10 between 5 and 10 years ago and 14 more than 10 years ago (Supplementary Appendix 2).
After conducting the quality assessment of the included papers and accounting for potential confounding bias associated with before and after studies, as well as the results from selection, information and reporting biases we conclude overall fairly low quality of evidence.
All reports were some form of ‘before-after comparison’, mostly with little formal description of methodology. The background settings within which the different interventions were tested varied considerably across the studies.
Characteristics of the interventions and the available outcome data
Of the 45 interventions identified, 13 targeted ethics review and 32 targeted governance processes (Table 1). The interventions were distributed across 12 countries and jurisdictions (Fig. 2). The country with the most interventions was India (1 ethics and 8 governance) followed by the UK (2 ethics and 6 governance). There were no interventions identified in Latin America or the Middle East. Only one intervention was identified for Europe though there were four reports about different aspects of that initiative.
The 13 ethics interventions comprised 4 interventions based on a single application model, 3 based on a mutual acceptance of review model, 2 based on the implementation of guidelines to standardise the application format, 2 based on streamlined approval and 2 others. The 32 governance interventions were 13 attempts to implement regulatory changes, 4 to implement a co-ordinating centre, 4 based upon a single application, 3 based on scope guidelines, 3 based on streamlining of the approval process and 5 others (Table 2).
There were 39/45 interventions for which there was a positive, null or adverse effect identified. The other 6 studies reported on the intervention form only (2 ethics (Care ACoSaQiH, 2020; Thompson, 2014) and 4 governance (Thompson, 2014; Madhani, 2010; Mani, 2006; Care ACoSaQiH, 2020), with no data on impact provided. Among the 39 interventions for which an outcome was recorded there was reporting on numbers of clinical trials for 38 (11 for ethics and 27 for governance) and expenditure on clinical trials for 5 (0 for ethics and 5 for governance).
Effects of interventions targeting ethics reform
Of the 11/13 attempts to reform ethics systems for which outcome data were available, 6 were positive (Care ACoSaQiH, 2020; Sarma, Manisha (2018); Nakamura et al., 2003; Thompson et al., 2009), 4 were null (Zannad et al., 2019; Industry CDo. 2011; Evans, Zalcberg (2016); Kong, 2007) and one was adverse (Warlow, 2005) (Table 3). The positive effects were mostly derived from interventions that implemented ‘scope guidelines’, placed ‘defined timeline’ expectations on review processes or established ‘mutual acceptance’ of review outcomes across ethics committees. For the four interventions reporting null effects this was attributed primarily to the interventions being of sound design but not being delivered with the fidelity intended (Zannad et al., 2019; Industry CDo., 2011; Evans and Zalcberg, 2016; Kong 2007). For example, the lack of enabling technology or infrastructure meant that the impact of the reforms was muted (Zannad et al., 2019). The adverse effect of an ethics intervention (Warlow, 2005) was observed in the United Kingdom and was attributed to the introduction of a new submission format, which the researchers found time consuming to complete and the ethics committees were incompletely equipped to assess. The defining characteristics that led to this negative result were a single centralised application process and an inadequate consideration of the wider research environment (Table 4).
Effects of interventions targeting governance reform
Of the 28/32 interventions targeting governance reform for which outcome data were available, seventeen (ATIC Australian Trade and Investment Commission, 2018; Caulfield, 2001; Zhang et al., 2015; Kong, 2007; Mossialos et al., 2016; Choudhury, Saberwal (2019); Srinivasan et al., 2009; McGee, 2006; Srinivasan, 2009; Ippoliti, Falavigna (2014); Konishi et al., 2018; Chen, 1998; Haffner, 1994; Care ACoSaQiH 2020; Sarma and Manisha, 2018) were positive, three were null (Fudge et al., 2010; Industry CDo., 2011) and eight were adverse (Van Oijen et al., 2017; Reith et al., 2013; Berge et al., 2015; Newman et al., 2016; Ikegami and Campbell, 1999; Warlow, 2005; Haynes et al., 2010; Hackshaw et al., 2008; Kwon and Jung, 2018; Hudson et al., 2016) (Table 3). The positive effects were mostly derived from two intervention strategies that overlapped with those effective in ethics review reform (‘scope guidelines’ and ‘defined timelines’). Scope guidelines limited the numbers of ambiguities in the process and fixed timelines held review bodies to defined schedules. Additionally, the introduction of ‘co-ordinating bodies’ that facilitated the governance review process across the various responsible organisations in a jurisdiction also delivered positive outcomes. Once again, the null governance interventions were considered primarily to be a consequence of failure to achieve uptake of the intervention as planned, rather than the intervention format being fundamentally flawed. The eight governance interventions reporting adverse effects were mostly initiatives based upon standardised protocols that were too proscriptive or resulted in duplication of effort. The European Union Clinical Trials Directive, for example, was intended to standardise governance processes with legislated EU-wide regulations. Ultimately the Directive was legislated in many countries but with differences across jurisdictions. The consequence was that multi-country clinical trials were required to understand and adhere to multiple different criteria across European Union sites with significant adverse implications for timelines and resources (Reith et al., 2013). The Directive was an example that contained each of the characteristics common amongst the negative results (i.e., a single centralised application process, inadequate consideration of wider research environment as well as a focus on retention of local control—Table 4).
Discussion
Governments have a clear opportunity to enhance clinical trial activity with interventions targeting ethics review and governance processes. However, the form of both ethics and governance interventions needs to be selected carefully to ensure they are effective. For both sets of interventions there were multiple examples of failures whereby no impact was achieved, and this appears mostly to have occurred because the interventions, while well-conceived, were not delivered as planned. There were also several examples of interventions that actually impeded clinical activity because the implemented interventions were not well designed (Table 4).
Interest in efficient clinical trial processes is increasing as governments around the world seek to capture the health and economic benefits of foreign and domestic research investment in their jurisdictions. India’s share of the global clinical trials market, for example, grew from 0.9 per cent in 2008 to 5 per cent in 2013 and China has experienced similar expansion as those countries took advantage of their large populations, rapidly developing workforce, and relatively low cost of business. At the same time, the share of clinical trial activities in the United States and other developed countries has been declining (Mondal and Abrol, 2015), spurring these more established markets to re-examine their own policy settings in an effort to retain valuable business.
Two forms of government intervention that were identified as more likely to be effective for both ethics and governance reform were the introduction of ‘scope guidelines’ and ‘defined timelines’. The former seeks to place clear boundaries around the breadth of the assessment required to be done by the ethics or governance agency and thereby achieve focus on the key actions required. Scope guidelines introduced in India in the early 2000s were credited with defining ambiguous topics and demarcating the responsibilities of sponsors, ethics committees and investigators, which resulted in enhanced throughput and increased numbers of approved trials (Sarma and Manisha, 2018). ‘Defined timeline’ interventions were primarily about placing clear targets on the acceptable maximum duration of each step in the passage of clinical trials though approval processes, with accompanying reporting on the timelines achieved. There is the potential that these amendments might erode or decrease the quality of decisions made and efforts by the Indian government have been criticised as such (Barnes et al., 2018). ‘Mutual acceptance’ interventions were also effective as an ethics reform measure (Care ACoSaQiH, 2020) and there was some evidence that the establishment of a central ‘co-ordinating body’ for the support of governance approval could bring benefits. The ‘co-ordinating body’ approach is importantly different to the ‘single application’ strategy, with the former seeking to facilitate governance processes across multiple entities, rather than trying to centralise all processes in a single body.
A theme central to multiple interventions was the intent of reduction of administrative burden. In general, this was viewed as a positive objective and where achieved was associated with positive outcomes. However, unintended effects sometimes resulted when programmes were not implemented as anticipated. The European Union sought to harmonise member state administration processes through the European Union Clinical Trials Directive (2001/20/EC) (European Union, Directive 2001/20/EC, 2001). Contrary to expectations, between 2003 and 2007, the average time from protocol finalisation to initiation of recruitment increased from 144 days to 178 days, rather than declining (Berge et al., 2015). Investigation revealed that in multiple jurisdictions Directive initiatives were layered on top of existing regulations rather than replacing them, because local ethics and governance bodies proved unwilling to divest responsibility to the Directive. This resulted in a more complex, variable and onerous system for clinical trialists to negotiate, which was exactly opposite to the goal intended. It was for these reasons that the directive was repealed by Regulation 536/2014 (European Union, 2014). The United States embarked on a similar effort to streamline the ethics review for multisite clinical trials (HHS UDoHaHS, 2017), which has left some commentators doubtful that the centralisation of the review process will allow ethics committees to guarantee the protection of research participants (Tusino and Furfaro, 2021).
Similarly common to interventions with adverse effects were interventions that had no effect, and while clinical trial activity was not reduced with these interventions there was an opportunity cost for each. The European and Developing Countries Clinical Trials Partnership and World Health Organization efforts to improve the administration of clinical trials throughout Africa are an example of a resource-intensive intervention with null effects. Poor clinical research infrastructure and suboptimal access to technology (Zannad et al., 2019) were identified as the primary causes of project failure.
The engagement of all relevant parties and a system-wide approach to enhancing clinical trial activity appears to be another factor important to success. There are several well-documented instances where one part of the system acting alone to introduce enhancements resulted in an adverse outcome. On several occasions processes introduced to improve patient safety or patient rights, (Ikegami and Campbell, 1999; Newman et al., 2016; Kwon and Jung, 2018) while having laudable objectives, failed the clinical trial system because of insufficient consultation. An inadequately consulted upon requirement that physicians alone could obtain consent for trial participation implemented in Japan did little to improve the quality of information received by trial participants but became a major new barrier to recruitment (Ikegami and Campbell, 1999). By contrast, ‘scope guidelines’ implemented in Japan following negotiation amongst researchers, ethics and governance bodies were deemed highly effective at removing ambiguities and accelerating review processes (Nakamura, 2003). In this latter case the full engagement of health administrators, researchers and research coordinators in a whole-of-system approach to the reforms was deemed central to their success. Governance interventions focused on retaining local administrative control were relatively common and were frequently associated with reduced clinical trial activity (Van Oijen et al., 2017; Warlow, 2005; Hackshaw et al., 2008; Haynes et al., 2010; Hudson et al., 2016).
While most countries enacted ethics or governance process changes to improve efficiency and reduce regulatory burdens, some countries utilised regulatory changes to implement powerful one-off interventions. China, for example, now requires that companies wishing to market their product in China include a given number of participants recruited locally within their clinical trial programmes (Zhang et al., 2015; Kong, 2007; Mossialos et al., 2016). The mandated inclusion of local study participants has likely been an important part of the decision by many large international companies to establish or grow their presence in China. In India, it was indirect action on the reform of national intellectual property safeguards that was central to encouraging foreign companies to establish a presence in India and do more local clinical trials (McGee, 2006).
Strengths and limitations
This review benefitted from the broad and systematic search of the literature done to try and capture all relevant information. The algorithms used by internet search engines can weight results towards user characteristics such as geography and language and this may have mitigated against the detection of reports from countries such as China and Korea—these are two markets that have significant clinical trial activity, that have implemented significant reforms but for which relatively few search results were returned. Additionally, most of the included studies were set in English-speaking jurisdictions and this may have been due to the exclusive use of English search terms and the algorithms. It is also possible that the search results were influenced by publication bias, which it was not possible to formally test for, given the limited data available across the constituent studies. Detail about the forms of intervention and nature of the evaluations were frequently sparse and categorising the interventions and outcomes was difficult as a consequence. For example, many studies referred only obliquely to ‘regulatory reforms’ meaning that large numbers of interventions were categorised non-specifically as ‘regulatory changes’. The inclusion of grey literature ensured that more relevant data were included but the quality of reporting was more varied, and this presented analytic challenges (Reith et al., 2013). It was also not possible to search every possibly relevant result returned from the grey literature searches because of the very large numbers. The standardised and duplicated extraction of information from the identified reports served to maximise the quality of the data that was available and the semi-quantitative approach to summarising information, nonetheless, provided for clearer insights than are possible from even a high-quality narrative review approach (Care ACoSaQiH, 2020). The studies came from only a small number of jurisdictions that are not representative of the globe though there was a mix of higher and lower-income countries included. Additionally, the study design may have omitted various interventions that did not include evaluations on the impact of numbers of clinical trials or relevant expenditures (such as legal acts). As such there is some uncertainty about the extent to which the main conclusions are generalisable across other countries, though it seems likely that key themes such as the reduction of bureaucracy and the need for effective implementation of selected interventions will be common across jurisdictions. Table 4 attempts to identify common characteristics to positive and adverse interventions despite these differences in culture, levels of development, health infrastructure and population types (Table 5).
Conclusion
Our data show that governments can pursue clinical trial reform programmes targeting ethics and governance processes with a reasonable expectation of increasing clinical trial activity and expenditure. Where governments achieve greater clinical trial activity there is also a reasonable expectation that the research sector, the health system, the community, and the economy will benefit and there is a high likelihood that the costs of reform processes will be offset. There is, however, also a clear risk that incompletely implemented reforms will fail and that poorly conceived programmes will make processes more onerous and reduce clinical activity.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
ATIC (Australian Trade and Investment Commission) (2018), Clinical Trials, Commonwealth of Australia. ATIC (Australian Trade and Investment Commission)
Barnes M, Flaherty J, Caron M, Naqvee A, Bierer B (2018) The evolving regulatory landscape for clinical trials in India. Food Drug Law J 4:601–623. https://www.jstor.org/stable/26826964
Berge E, Ford GA, Bath PM, Stapf C, van der Worp HB, Demotes J et al(2015) Regulation and governance of multinational drug trials in stroke: barriers and possibilities. Int J Stroke 10(3):425–428
Care ACoSaQiH (2020) The National Clinical Trials Governance Framework Literature review. Care ACoSaQiH
Caulfield T (2001) Globalization, conflicts of interest and clinical research: an overview of trends and issues. Wide Law Symp J 8(31):31–45
Chen KC (1998) ICH in Taiwan. Therapeutic innovation and regulatory science 32(1):1301S–1305SS. https://doi.org/10.1177/00928615980320S124
Choudhury MC, Saberwal G (2019) The work, goals, challenges, achievements, and recommendations of orphan medicinal product organizations in India: an interview-based study. Orphanet J Rare Dis. 14(1). https://ojrd.biomedcentral.com/articles/10.1186/s13023-019-1224-0
Chengodu T (2013) A qualitative study exploring the role of clinical trials nurses in Australia. University of Sydney
Committee UHoCSaT (2013) Clinical trials: third report of session 2013-2014. Committee UHoCSaT
DOH (Department of Health), (2021) Clinical trials, https://www1.health.gov.au/internet/main/publishing.nsf/Content/Clinical-Trials
European Union, Directive 2001/20/EC (2001) of the European Parliament and of the Council on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. European Parliament
European Union (2014) Regulation of the European Union on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. European Parliament
Evans S, Zalcberg J (2016) Enough is enough… a call to action to improve ethical and governance review processes in Australia. Int Med J 46(12):1362–1364
Fudge N, Redfern J, Wolfe C, McKevitt C (2010) Streamlined research governance: are we there yet? BMJ 341:4624
Hackshaw A, Farrant H, Bulley S, Seckl MJ, Ledermann JA(2008) Setting up non-commercial clinical trials takes too long in the UK: findings from a prospective study. J R Soc Med 101(6):299–304. https://journals.sagepub.com/doi/10.1258/jrsm.2008.070373
Haffner ME(1994) Applications of the orphan drug act to special patient populations. Ther Innov Regul Sci 28(2):495–503 https://journals.sagepub.com/doi/abs/10.1177/009286159402800224
Haynes R, Bowman L, Rahimi K, Armitage J (2010) How the NHS research governance procedures could be modified to greatly strengthen clinical research. Clin Med 10(2):127
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2021) Cochrane handbook for systematic reviews of interventions version (updated February 2021). 6.2 edn. Cochrane
Hudson KL, Lauer MS, Collins FS (2016) Toward a new era of trust and transparency in clinical trials. JAMA 316(13):1353–1354
HHS (UDoHaHS) (2017) Federal policy for the protection of human subjects. HHS (UDoHaHS)
Ikegami N, Campbell JC (1999) Health care reform in Japan: the virtues of muddling through: tops in equality of access, among the lowest in health spending, Japan nevertheless has important problems to solve—gradually. Health Aff 18(3):56–75
Ippoliti R, Falavigna G (2014) Public health institutions, clinical research and protection system of patients’ rights: an impact evaluation of public policy. Public Organiz Rev 14(2):109–125
Industry CDo. (2011) Clinically competitive: boosting the business of clinical trials in Australia. Clinical Trials Action Group Report. Industry CDo
Kong L (2007) Clinical trial opportunities in China. Appl Clin Trials 16(4):58-,60,2,4,6. PubMed PMID: 201517623
Konishi A, Isobe S, Sato D (2018) New regulatory framework for medical devices in Japan: current regulatory considerations regarding clinical studies. J Vasc Intervent Radiol 29(5):657–660. https://doi.org/10.1016/j.jvir.2017.12.022
Kwon H, Jung E-Y (2018) The impact of policy on the growth of precision medicine. Health Policy Technol 7(4):347–357
Madhani PM (2010) Clinical and contract research: potential. J Indian Manag. October–December 6:77–88
Mani S (2006) The sectoral system of innovation of Indian pharmaceutical industry. CDS working papers, no. 382
McGee P (2006) Clinical trials on the move. Drug Discov Dev 9(6):16–22
Mondal S, Abrol D (2015) Clinical trials industry in India: a systematic review: Institute for Studies in Industrial Development New Delhi
Mossialos E, Ge Y, Hu J, Wang L, (2016) Pharmaceutical policy in China: challenges and opportunities for reform. World Health Organization. Regional Office for Europe
McKeon S, Alexander E, Brodaty H, Ferris B, Frazer I, Little M (2013) Strategic Review of Health and Medical Research - Better Health Through Research, Department of Health and Ageing, Australian Government, Canberra
Nakamura T, Yamamoto K, Nagai R, Horiuchi R(2003) Content and classification of clinical trials at a university hospital in Japan Jpn Heart J. 44(2):235–242. https://pubmed.ncbi.nlm.nih.gov/12718485/
NHMRC (National Health and Medical Research Council), Australian Clinical Trials, (2021) https://www.australianclinicaltrials.gov.au/what-clinical-trial
Newman C, Ajay VS, Srinivas R, Bhalla S, Prabhakaran D, Banerjee A (2016) Drugs for cardiovascular disease in India: perspectives of pharmaceutical executives and government officials on access and development-a qualitative analysis. J Pharmaceut Policy Pract 9(1):1–11
Reith C, Landray M, Devereaux PJ, Bosch J, Granger CB, Baigent C, Califf RM, Collins R, Yusuf S (2013) Randomized clinical trials–removing unnecessary obstacles. New Engl J Med 369(11):1061
Sarma KAS, Manisha (2018) Clinical Trials in India. Food and Drug Law Institute
Srinivasan S, Nikarge S (2009) Ethical concerns in clinical trials in India: an investigation. Centre for Studies in Ethics and Rights Mumbai
Srinivasan S (2009) The clinical trials scenario in India. Economic and Political Weekly, 29–33
Thompson SC, Sanfilippo FM, Briffa TG, Hobbs MS (2009) Towards better health research in Australia—a plea to improve the efficiency of human research ethics committee processes. Med J Aust 190(11):652
Thompson S (2014) To Market, To Market: Ontario, Canada’s vision to improve commercialization of healthcare research: Research and Regulation. J Comm Biotechnol 20(4). https://doi.org/10.5912/jcb660
Tusino S, Furfaro M (2021) Rethinking the role of Research Ethics Committees in the light of Regulation (EU) No. 536/2014 on clinical trials and the COVID-19 pandemic. Br J Clin Pharmacol 88(1):40–46
Van Oijen JC, Grit KJ, van de Bovenkamp HM, Bal RA (2017) Effects of EU harmonization policies on national public supervision of clinical trials: a dynamic cycle of institutional change and institutional work. Health Policy 121(9):971–977
Warlow C (2005) Over-regulation of clinical research: a threat to public health. Clin Med 5(1):33
Webster SM, Temple-Smith M (2013) The red tape waltz. Monash Bioeth Rev 31(1):77–98
Whitney SN, Schneider CE (2011) A method to estimate the cost in lives of ethics board review of biomedical research. J Int Med 269(4):396–402
Zannad F, Sobhy M, Almahmeed W, Balghith M, Butler J, Dziri S (2019) Clinical research in Africa And Middle East: roadmap for reform and harmonisation of the regulatory framework and sustainable capacity development. J Global Health Rep 3:e2019082
Zhang M-Y, Li J, Hu H, Wang Y-T (2015) Seizing the strategic opportunities of emerging technologies by building up innovation system: monoclonal antibody development in China. Health Res Policy Syst 13(1). https://doi.org/10.1186/s12961-015-0056-1
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crosby, S., Rajadurai, E., Jan, S. et al. The effects of government policies targeting ethics and governance processes on clinical trial activity and expenditure: a systematic review. Humanit Soc Sci Commun 9, 266 (2022). https://doi.org/10.1057/s41599-022-01269-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1057/s41599-022-01269-3