Abstract
Early diagnosis of Brucellosis is often difficult in the patient with only single non-specific symptom because of its rarity. We report a patient with Brucellar spondylodiscitis, in which the low back pain was the only symptom and the magnetic resonance imaging (MRI) showed not radiographic features about infection at initial stage. He was misdiagnosed as a lumbar disc herniation for inappropriate treatment in a long time. The delay in diagnosis and correct treatment led to rapid progression of the disease and severe complications. The patient was treated successfully with triple-antibiotic and surgical intervention in the end. Brucellar spondylodiscitis should always be suspended in the differential diagnosis specially when the patient comes from an endemic area or has consumed dairy products from animals in such an area and comprehensive examination should be done for the patent to rule out some important diseases like Brucellosis with sufficient reasons.
Similar content being viewed by others
Brucellosis is caused by small, non-motile, Gram-negative, aerobic and facultative intracellular coccobacilli of the genus Brucella transmitted from infected animals to humans either by consumption of unpasteurized milk or dairy products or by direct contact with infected tissue.1–14 Human brucellosis, an occupational disease, is more widespread among farmers, veterinarians and laboratory workers or animal breeders.1 Common symptoms include fever, fatigue, arthralgias, myalgias, sweat, decreased appetite, weight loss, and back and low back pain.1–4 The bone-joint involvement is the most common complications, especially sacroilitis and spondylitis, which can result in Brucella-related spinal epidural abscesses (SEA) commonly seen in the thoracic or lumbar spine regions, and, less frequently in the cervical spine region as localized abscesses.2,3 There are several case reports and series about Brucellar spondylodiscitis with epidural abscesses presenting with other myelopathy or neuropathy in the published literature, but not with cauda equina syndrome. SEA rarely give rise to spinal cord compression in spinal, particularly presenting with cauda equina syndrome because of a large space of the lumbar vertebral canal. Herein, we report a case of lumbar Brucellar spondylodiscitis with rapidly progressive SEA presenting with cauda equina syndrome, to strengthen the awareness of this disease.
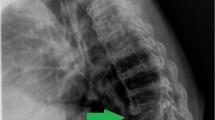
A 52-year-old male quarantine inspector was admitted to our hospital with a history of low back pain persisting for 3 months and left lower limb numbness beginning 3 weeks earlier. One month before admission, he went to the local clinic because of the low back pain. After an MRI of the lumbar spine showed the intervertebral disc herniation of L4–5, he received medical treatment with nonsteroidal anti-inflammatory drugs and myorelaxants. He reported the use of medical therapeutic regimen with no improvement. His pain was gradually increased in recent weeks, and was worse when sitting or walking for long periods of time. He also complained of irregular fever and sweat at post meridiem and intermittent left lower limb numbness for the recent weeks. One week before admission, the patient returned to the local clinic because his symptoms worsen. Computed tomography (CT) showed periosteal proliferation at the L4 vertebral body and bone destruction with osteophytic formation at the L5 vertebral body (Figure 1). Laboratory investigations showed high levels of c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). The Brucella seroagglutination test was positive in a titer of 1:200. Sagittal T2-weighted MR image revealed hypointense signals in L5 vertebrae, indicating active infectious spondylodiscitis and cartilage endplate involvement, but the spinal cord showed normal signal intensity (Figure 2). The primary diagnosis was brucella spondylodiscitis from L4 vertebra to L5, and he was treated with combination antibiotics with tetracycline and rifampicin. His symptoms was unresponsive to the therapeutic regimen for several days, and then he was transferred to our hospital for further evaluation and treatment.
On examination at our hospital, the patient’s temperature was 38.6 °C, pulse was 112 per min, respiratory rate was 20 per min and blood pressure was 110 per 74 mm Hg. There was severe low back pain and limitation in the motion of the back in all directions. He showed moderate tenderness to palpation in the L4–5 area and bilateral paraspinal muscle contraction. There was decreased sensation in the left calf. Muscle strength testing and deep tendon reflex examination were normal. Initial laboratory studies revealed ESR of 70 mm h−1 (0–15 mm h−1, male), CRP of 231 mg l−1(0–8 mg l−1), white blood cells count of 6.9×109 g l−1 (3.7–10 g l−1), neutrophil granulocyte ratio of 87.9% (43–75%) and lymphocyte percentage of 8.9% (17–43%). Tests for antistreptolysin-O antibodies, tuberculin-skin and rheumatoid factor were negative. The culture of blood specimens, obtained on admission, was negative for 1 week and the Brucella standard tube-agglutination test was positive in a titer of 1:320. L-spine anteroposterior and lateral plain radiographs showed that there were some osteophyte formations at L5 vertebrae and the disc spaces were almost normal. The fever type of the patient was undulant fever, and the highest temperature was no >39.5 °C. The patient was treated with triple-antibiotic (doxycycline 100 mg b.i.d. orally plus rifampicin 600 mg q.d. orally plus levofoxacin 300 mg b.i.d. intravenous drip). After the patient’s temperature returned to normal, the patient underwent fluoroscopy-guided percutaneous puncture biopsy of the L5 vertebrae lesion for histopathologic examination and tissue specimen cultures to confirm the pathogenic bacteria. The patient went on with triple-antibiotic treatment.
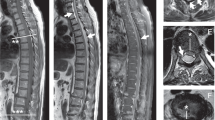
Three days after biopsy, the patient suddenly presented with lower limb weakness, walk flabby, sciatica, saddle anesthesia and urinary retention. Physical examination showed that there were pareses with 3/5 muscle strength in both lower extremities, ankle reflex absent on both sides and absent anal reflex. The repeat MRI demonstrated L4–5 spondylodiscitis and epidural abscesses extending to prevertebral region and epidural space causing cauda equina compression. The T1-weighted images of epidural abscesses were hypointense signals, but the signals in these areas became hyperintense on T2-weighted sequences (Figures 3 and 4). Brucellar spondylodiscitis complicated by cauda equina syndrome was a suspected diagnosis based on the serological, radiological and clinical findings. The patient was taken to the immediate operation by the Wiltse paraspinal approach and implantation of pedicle screws were done. Then bilateral partial facetectomy and laminectomy were done to allow visualization and removal of the intervertebral tissues and epidural abscess. The neutral tissue was decompressed completely by the evacuation of the epidural abscess and removal of the inflammatory tissue after collecting gray-yellow colored samples for pathology and culture. A large amount of normal saline was used to intraspinal irrigation so that there was no residual inflammatory tissues. Autogenous iliac bone grafts mixed with vancomycin (1g) and single ‘banana’-shaped spacer were used for fusion. The pedicle screws are then connected with metallic rods and fluoroscopic X-ray showed that the spacer and fixation devices in the good position (Figure 5). Tissue specimen cultures were positve for Brucella melitensis, thus, the final diagnosis was Brucellar spondylodiscitis with cauda equina syndrome. Neurological signs and symtoms of the patient were gradually recovered from the surgery several days later. The triple-antibiotic treatment (doxycycline 100 mg b.i.d. orally) plus rifampicin (450 mg q.d. orally) plus levofoxacin(150 mg q.d. orally) was went on for 8–12 weeks, but the specific duration depends on the clinic response. The contents of periodical follow-up in out-patient clinic include blood routine, ESR, CRP, renal and liver functions, plain radiographs and even MRI.
Brucellosis is a zoonosis, which is still a common public health problem mainly in the Mediterranean region, the Middle East and the South America.1–3 However, it is also endemic in the Northwest, the Northeast, the Qinghai-Tibetan Plateau and the Inner Mongolia of the China that are mainly nomdic zones.4 Brucellosis is one of category B infectious diseases by provision of the law of the infections in China. The main transmission routes of spine are haematogenous spread, direct external inoculation or involvement from contiguous tissue.15,16 Haematogenous spondylodiscitis occurs in predominantly the lumbar spine, followed by the thoracic, the cervical spine and a multifocal involvement very rarely.1,3 In general, the upper cartilage endplate of vertebrae is involved at first because of its rich blood supply, and then the whole vertebra, intervertenral space and adjacent vertebrae may be involved.1,3 The sheep and cattle of endemic areas are the main infection sources in China, which are transmitted commonly by broken skin or mucosa or conjunctiva, or through ingeation or in inhalation.4,5 Skin, upper respiratory tract infection and osteomyelitis of adjacent vertebrae are the main sources of SEA, but the most probable source of Brucella-ralated SEA is osteomyelitis of adjacent vertebrae.3,6 Our patient, living in the forementioned endemic area, reported occupational exposure to all kinds of animals as a quarantine inspector such as cattle, sheep and pig, and the specific contamination route could be direct contact. Spinal involvement in brucellosis, the significant cause of the debilitating and disabling complications, occurs in 6–12% of cases.7,16 Brucellar spondylodiscitis, extending to the prevertebral region and epidural spaces, is a rare complication in spinal brucellosis.3 As Ulu-Kilic et al. studied, Brucellar spondylodiscitis was complicated, with paravertebral abscess in 13.0% of patients, prevertebral abscess in 4.4%, epidural abscess in 10.2%, psoas abscess in 3.4% and radiculitis in 2.7%.16 Up to date, there are only two case reports about Brucellar spondylodiscitis with SEA rapidly developing incomplete paraplegia in the literature of the China.4,5 Our patient underwent L4–5 spondylodiscitis and epidural abscesses extending to prevertebral region and epidural space causing spinal cord compression, presenting with cauda equina syndrome. There are also not similar reports exiting in the published literatures. Although there exists a large space in the lumbar vertebral canal, the SEA of Brucellar spondylodiscitis can still compress the cauda equine and lead to severe complication.
The symptoms of Brucellar spondylodiscitis are non-specific including fever, fatigue, polyarthromyalgia, sweat, decreased appetite, weight loss, and neck or low back pain.1–4 The majority of patients complain of neck or low back pain, but up to 15% of cases may be absent; fever is far less common and only about 50% of cases present with fever.8 However, the outstanding spinal pain, fever and local tenderness are the main clinical manifestations of SEA.3 In the study by Safak Kaya et al., back or neck pain was the most common symptom, up to 97.2% of patients, and fever was present in 28.9%, neurologic abnormalities in 12.1%.9 On the basis of the duration of symptom, brucellosis was classified as acute (<12 months), subacute (2–13 momths) and chronic(>13 months) brucellosis.2 Our patient, whose symptomatic duration was 3 months, belonged to subacute brucellosis. The low back pain was the only symptom for the fist 2 months, by which few clinicians can take the disease of Brucellosis into consideration and the misdiagnosis occurs commonly. The patient began to present with the fever and sweat until the final phase of duration of symptom. Our patient, being very interesting, rapidly developed into SEA within about 1 week under the condition of intensive therapy, indicating higher virulence once the disease is at active phase.
It is important to make a correct early diagnosis of brucellosis because this disease is only too aggressive to require immediate treatment. Difficulties in the early diagnosis of Brucellar spondylitis are that the symptoms are non-specific, particularly presenting only one symptom just like our patient. Blood culture is a very important diagnostic tool in brucellosis, but the positive results are in 21–56% of patients because of the intermittent bacteraemia and the timing of the culture.3,10,16 Serology tests are cost-effective tools, but It should be noted that serology tests may be negative at first day or week and become positive with the development of the disease. There also exist some case reports in which serology tests were persistently negative.11 Plain radiographs may be useful because of radiographic features taking several months to occur and radiographic changes may be difficult to distinguish between infection destruction and degenerative changes because of bony remodeling.12 Radionuclide tests are seldom used currently, however, fluorine-18 FDG-PET (fluorodeoxyglucose positron emission tomography) may be a complementary method for determining the efficacy of treatment because it is correlated with a significant decrease in SUVmax (maximum standardized uptake value) values if treatment is successfull and can provide additional information on the spread of the infection compared with MRI.13 MRI is considered to be good choice for detecting the early spondylodiscitis because of its high sensitivity, and provides excellent definition of paravertebral and epidural extension particularly with gadolinium enhancement.3,14 CT-scan can give significant information on bone destruction, which is particularly beneficial to surgical planning.2,3
The combination of antimicrobial drugs should be used to treat brucellosis if the diagnosis is definite. Early Intensive therapy of Brucellar spondylodiscitis is beneficial to preventing devastating complications. According to the clinical features of the patient, such as age, side-effects and physical status, selection of an appropriate antibiotic combination is significant. We should take surgical treatment into consideration when there are neurological deficits resulting from the compression of nerve root or spinal cord.1,3 Our patient was treated actively with triple-antibiotic at the initial stage, but immediate surgery was arranged for the patient because of cauda equine compression resulting in paraparesis. Specific morbid conditions decide selection of the most appropriate procedures, including less invasive techniques and classic open surgery (anterior, posterior and combined types). Our patient chose the transforaminal lumbar interbody fusion by the Wiltse paraspinal approach based on the following reasons: (1) this approach, entering through the space between the multifidus and the longissimus, and reserving the innervation and blood supply of sacrospinalis because of avoiding dissecting it, can avoid the postoperative low back pain resulting from atrophy in sacrospinalis; (2) suture of muscle space can form the complete barrier to prevent the formation of sinus from the diffusion of the front lesion; (3) Preserving the integrity of posterior structures of spinal canal can reduce dural sac and nerve root adhesion because of substantially lamina removal and also prevent the diffusion of lesion; (4) In general, this approach can reduce intraoperative injury and bleeding.
Brucellar spondylodiscitis with SEA is rare, which can lead to severe complications and even death if not treated timely. In the face of single non-specific symptom, clinicians have difficulty in making a correct early diagnosis of Brucellar spondylodiscitis. For some patients who come from endemic areas with non-specific symptom mimicking brucellosis, some important examinations, such as MRI and serologic tests should be done to rule out this disease.
References
Turgut M, Turgut AT, KoŞar U . Spinal brucellosis:Turkish experience based on 452 cases published during the last century. Acta Neurochir (Wien) 2006; 148: 1033–1044. discussion 1044.
Ulu-Kilic A, Metan G, Alp E . Clinical presentations and diagnosis of brucellosis. Recent Pat Antiinfect Drug Discov 2013; 8: 34–41.
Koubâa M, Mâaloul I, Marrakchi Ch, Lahiani D, Znazen A, Hammami B et al. Brucellar spinal epidural abscesses.Single center experience of nineteen patients and review of the literature. Egypt Rheumatol 2013; 35: 15–20.
Fei Q, Yang Y, Li JJ, Li D, Wang BQ . Brucellar spondylodiscitis resulting in incomplete paralysis of the lower extremities: one case report and review of the literature (in Chinese). China J Clin 2014; 2: 48–50.
Zhao MY, Lu Y, Wang RQ, Lv J, Xiao XG . Brucellar spondylodiscitis causing spinal cord compression: a case report [in Chinese]. Chin J Nerv Ment Dis 2015; 1: 60–61.
Reihsaus E, Waldbaur H, Seeling W . Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev . 2000; 23: 175–204. discussion 205.
Ulu-Kilic A, Sayar MS, Tutuncu E, Sezen F, Sencan I. Complicated brucellar spondylodiscitis: experience from an endemic area. Rheumatol Int 2013; 33: 2909–2912.
Pradilla G, Nagahama Y, Spivak AM, Bydon A, Rigamonti D . Spinal epidural abscess: current diagnosis and management. Curr Infect Dis Rep 2010; 12: 484–491.
Kaya S, Ercan S, Kaya S, Aktas U, Kamasak K, Ozalp H et al. Spondylodiscitis: evaluation of patients in a tertiary hospital. J Infect Dev Ctries 2014; 8: 1272–1276.
Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H . A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect 2007; 55: 158–163.
Song KJ, Yoon SJ, Lee KB . Cervical spinal brucellosis with epidural abscess causing neurologic deficit with negative serologic tests. World Neurosurg 2012; 78: 375.e15–e9.
Koubaa M, Maaloul I, Marrakchi C, Lahiani D, Hammami B . Spinal brucellosis in South of Tunisia: review of 32 cases. Spine J 2014; 14: 1538–1544.
Ioannou S, Chatziioannou S, Pneumaticos SG, Zormpala A, Sipsas NV . Fluorine-18 fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan contributes to the diagnosis and management of brucellar spondylodiskitis. BMC Infect Dis 2013; 13: 73.
Chelli Bouaziz M, Ladeb MF, Chakroun M, Chaabane S . Spinal brucellosis: a review. Skeletal Radiol 2008; 37: 785–790.
Gouliouris T, Aliyu SH, Brown NM . Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010; 65 (Suppl. 3): iii11–iii24.
Ulu-Kilic A, Karakas A, Erdem H, Turker T, Inal AS, Ak O et al. Update on treatment options for spinal brucellosis. Clin Microbiol Infect 2014; 20: O75–O82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hu, T., Wu, J., Zheng, C. et al. Brucellar spondylodiscitis with rapidly progressive spinal epidural abscess showing cauda equina syndrome. Spinal Cord Ser Cases 2, 15030 (2016). https://doi.org/10.1038/scsandc.2015.30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2015.30
This article is cited by
-
Noncontiguous multifocal Brucella spondylodiscitis with paravertebral abscess: a case report
Journal of Medical Case Reports (2022)