Abstract
Study design:
Randomized experimental study.
Objectives:
The study aimed to investigate the therapeutic efficacy and molecular mechanisms of A-68930 in a rat model of spinal cord injury (SCI)–induced acute lung injury (ALI).
Setting:
China.
Methods:
The influences of A-68930 on the pulmonary edema, histological changes, proinflammatory cytokines levels, myeloperoxidase (MPO) activity and NLRP3 inflammasome protein expression were estimated.
Results:
SCI significantly promoted NLRP3 inflammasome activation, increased proinflammatory cytokine productions and MPO activity, and induced pulmonary edema and tissue damage in the SCI group as compared with the control group. A-68930 administration significantly inhibited NLRP3 inflammasome activation and reduced inflammatory cytokines levels and MPO activity. Moreover, A-68930 administration attenuated pulmonary edema and histopathology.
Conclusion:
Our experimental findings indicated that A-68930 exhibited a protective effect on SCI-induced ALI by the alleviations of inflammatory response with the inhibition NLRP3 inflammasome activation 72 h post injury. The present study indicated that A-68930 could be a potentially efficient therapeutic strategy for the treatment of SCI-induced ALI.
Similar content being viewed by others
Introduction
Traumatic spinal cord injury (SCI) is a disastrous event that can lead to systemic inflammatory response syndrome and secondary multiple organ complications.1, 2, 3 Pulmonary complications are reported to be one of the major causes of death in SCI patients.4, 5, 6 Inflammation is a crucial mechanism in the pathophysiology of SCI-induced acute lung injury (ALI).7 Targeting of the inflammatory response can exert protective effects in SCI-induced ALI in rats.2, 7 Despite a number of trials about the relevant pathogenesis, there is a lack of effective medicine for the treatment of ALI.8 Hence, it is urgent to explore the efficient therapeutic strategy.
Pulmonary inflammatory responses include the maturation and secretion of proinflammatory cytokines interleukin (IL)-1β and IL-18, which induce an increase in endothelial permeability.9 The maturation and secretion of pro-IL-1β and pro-IL-18 require the activation of proteolytic enzyme caspase-1, which is mediated by the activation of nucleotide-binding domain-like receptor protein 3 (NLRP3) and subsequently the recruitment of apoptosis-associated speck-like protein (ASC).10, 11 The NLRP3 inflammasome, a kind of cytosolic protein signaling complex, consists of NLRP3, ASC and caspase-1 and is assembled after endogenous 'danger'.12, 13 The NLRP3 inflammasome regulates the maturation and release of IL-1β and IL-18;14, 15, 16 targeting of the NLRP3 inflammasome can exert protective effects in paraquat9 and lipopolysaccharide (LPS)17-induced ALI.
A-68930, a specific and a potent dopamine D1 receptor (DRD1) agonist, exhibits sedative,18 neuroprotective, anti-stress19 and anti-inflammatory20 effects. DRD1 has been proved to be present in almost all immune effector cells including bone marrow-derived macrophages, microglias, astrocytes, normal human leukocytes and dendritic cells.20, 21, 22 Dopamine suppresses NLRP3 inflammasome activation via DRD1 signaling, and DRD1 signaling induced NLRP3 ubiquitination and autophagy-mediated degradation.20 DRD1 signaling mitigates LPS-induced systemic inflammation and monosodium urate crystal-induced peritoneal inflammation by inhibition of NLRP3 inflammasome activation.20 Recent study has shown that A-68930 significantly controls neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation and reduces remarkable loss of neurons by suppressing NLRP3 inflammasome activation in mice.20 However, no study has been performed to investigate the therapeutic efficacy and molecular mechanisms of A-68930 in SCI-induced ALI in rats.
On the basis of above considerations, we investigate whether NLRP3 inflammasome is involved in SCI-induced ALI and whether A-68930 could inhibit NLRP3 inflammasome activation with protective effects in a rat model of SCI-induced ALI.
Materials and methods
Animals and groups
Adult female Sprague-Dawley rats weighing 250–300 g were purchased from Beijing Haidian Thriving Experimental Animal Centre (Beijing, China). All procedures for these experiments complied with the guidelines of the Animal Ethics Committee of Hangzhou First People's Hospital (Hangzhou, China). Rats were housed in a standard animal room with a 12 h light/dark cycle.
Eighty rats were randomly assigned into four equal groups via a random number table: (i) control group, where rats were uninjured; (ii) SCI group, where rats underwent SCI; (iii) SCI+Vehicle (Veh) group, where rats were intraperitoneally injected with 1 ml vehicle (0.1% propylene glycol in 1 ml sterile saline) immediately after SCI; and (iv) SCI+A-68930 group, where rats were intraperitoneally injected with 5 mg kg−1 A-68930 (Sigma–Aldrich, St Louis, MO, USA) in a 1 ml vehicle immediately after SCI. All animals in the SCI+A-68930 and SCI+Veh groups were intraperitoneally injected with an equal volume of the 5 mg kg−1 A-68930 or vehicle at 8-h intervals for 3 days (9 times totally). The dose and timing of A-68930 were based on previous study.20
Surgical procedure
SCI was induced by a model described by Young.23 Rats were anesthetized with an intraperitoneal injection of 3.0 ml kg−1 10% chloral hydrate. Laminectomy was performed to expose the spinal cord at the vertebral T9–T11 segment without damage to the dura. The spinal cord at the vertebral T10 segment (spinal T9) underwent a 25 g·cm impact. Rats were administered intramuscular injections of penicillin (400 000 unit per animal per day) and buprenorphine to prevent infection and relieve pain postoperatively. In addition, rats underwent manual bladder emptying twice a day. All rats were killed 72 h post injury on the basis of our preliminary experiment.
Pulmonary permeability index (PPI) and lung wet weight-to-dry weight (W/D) ratio
PPI and lung W/D were assessed on the basis of the method recorded by Gao et al.24 72 h after injury. The protein level in bronchoalveolar lavage fluid (BALF) and plasma was quantified by the Bicinchoninic Acid Protein Assay kit (Pierce, Rockford, IL, USA). The ratio of BALF protein/plasma protein (which is regarded as the PPI) was used to assess pulmonary vascular permeability in the airway (n=5 in each group).
Lungs were removed, weighed and subsequently dried in an oven at 60 °C for 72 h. The purpose of lung W/D was to evaluate the extent of pulmonary edema (n=5 in each group).
Histological study
Seventy-two hours after injury, rats (n=5 in each group) were perfused with 0.9% saline and subsequently with 4% paraformaldehyde. For the histologic analyses, some paraffin lung sections were stained with hematoxylin-eosin reagent. Histologic scoring was based on (i) edema, (ii) inflammatory cell infiltration, (iii) congestion and (iv) hemorrhage, and the score of each item was recorded as follows: 0, normal; 1, mild; 2, moderate; and 3, severe.25
Biochemical analysis
After the BALF samples were obtained 72 h after injury, they were immediately centrifuged at 2000 r.p.m. for 15 min at 4 ° C. IL-1β, IL-18 and tumor necrosis factor (TNF)-α concentrations in the collected supernatants were determined through enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA).
Measurement of MPO activity
Myeloperoxidase (MPO), a particular oxidative enzyme, widely exists in neutrophil granules and acts as a specific marker of neutrophil infiltration and activation. The measure of MPO activity in the lung tissue was performed in the lung tissue 72 h after injury with a commercial kit (Jiancheng Co, Nanjing, China) according to the manufacturer’s instructions (n=5 in each group).
Extraction of the protein and western blot analysis
The lung samples were removed 72 h after injury and stored at −80 °C until use. Specimens were homogenized in RIPA buffer and then centrifuged at 12 000 r.p.m. for 30 min at 4 °C. Protein concentration in the supernatant was quantified via the Bicinchoninic Acid method. Total protein (20 μg) was separated with 10% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% skimmed milk and subsequently incubated with specific primary antibodies overnight at 4 °C. Primary antibodies contained anti-NLRP3, anti-ASC, anti-caspase-1 and anti-β-actin (all 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing with phosphate buffered saline twen (PBST), the membranes were incubated with a horseradish peroxidase-coupled secondary antibody (1:1000; Millipore). Detection of proteins was performed using the enhanced chemiluminescence kit (Thermo Scientific, Rockford, IL, USA). Protein levels were analyzed via imaging software (Quantity One; Bio-Rad Co. Ltd., Hercules, CA, USA).
Statistical analysis
Data in the study are expressed as the mean±s.e. of the mean (s.e.m.) and were analyzed using SPSS software version 16.0 (SPSS Inc, Chicago, IL, USA). Comparisons between different animal groups were performed by one-way analysis of variance and the Dunnett post hoc test. A P-value of less than 0.05 was considered to be statistically significant.
Results
A-68930 administration reduces pulmonary vascular permeability and edema
The PPI and W/D ratio were used for assessing the influence of A-68930 on the pulmonary vascular permeability and edema, respectively, in SCI-induced ALI rats. Our result showed that the PPI (Figure 1a; P<0.001) and W/D ratio (Figure 1b; P<0.01) were significantly increased in the SCI group as compared with the control group. Furthermore, there was no significant difference between the SCI and the SCI+Veh groups with regard to PPI and W/D ratio. In comparison with the SCI+Veh group, rats in the SCI+A-68930 group showed a significant decrease in PPI (Figure 1a; PPI: P<0.01) and W/D ratio (Figure 1b; W/D ratio: P<0.05).
A-68930 administration reduces histopathologic damage
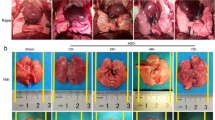
Histopathologic changes of the lung tissue were used for estimating the protective effects of A-68930 on rats with SCI-induced ALI. Compared with no change in the control group (Figure 2a), congestion, edema and structural disruption were shown in the SCI group (Figure 2b; P<0.001). Nevertheless, the changes were inhibited significantly by A-68930 administration in the SCI+A-68930 group (Figure 2d; P<0.01). Histopathological scores were calculated and are shown in Figure 2e.
Effects of A-68930 on histopathologic change in the lung tissue 72 h after SCI. ***P<0.001 compared with the control group; ##P<0.01 compared with the SCI+Veh group. The data represent means±s.e.m. (a) control group; (b) SCI group; (c) SCI+Veh group; (d) SCI+A-68930; (e) histopathologic scores. A full color version of this figure is available at the Spinal Cord journal online.
A-68930 administration reduces proinflammatory cytokine levels
SCI significantly increased proinflammatory cytokines IL-1β, IL-18 and TNF-α productions in the SCI group (Figure 3a, IL-1β: P<0.001; Figure 3b, IL-18: P<0.01; Figure 3c, TNF-α: P<0.001) as compared with the control group. Furthermore, there was no significant difference between SCI and SCI+Veh groups with regard to IL-1β, IL-18 and TNF-α levels. However, A-68930 administration significantly reduced the change of proinflammatory cytokines levels in the SCI+A-68930 (Figure 3a, IL-1β: P<0.01; Figure 3b, IL-18: P<0.05; Figure 3c, TNF-α: P<0.05) group as compared with the SCI+Veh group.
A-68930 administration inhibits MPO activity
In comparison with the control group, the MPO activity in lung tissue (Figure 4, P<0.05) increased significantly in the SCI group. Furthermore, there was no significant difference between the SCI and the SCI+Veh groups. Nevertheless, A-68930 administration in the SCI+A-68930 group (Figure 4, MPO activity: P<0.05) group significantly inhibited MPO activity as compared with the SCI+Veh group.
A-68930 administration inhibits NLRP3 inflammasome activation
SCI induced significant upregulated protein expression of NLRP3, ASC and active-caspase-1 in the SCI group (Figure 5, NLRP3: P<0.001; ASC: P<0.01; active-caspase-1: P<0.001) as compared with the uninjuried group. Furthermore, there was no significant difference between the SCI group and the SCI+Veh group with regard to NLRP3, ASC and caspase-1 levels. However, A-68930 administration significantly reduced the NLRP3 and active-caspase-1 levels in the SCI+A-68930 (Figure 5, NLRP3: P<0.01; active-caspase-1: P<0.01) group as compared with the SCI+Veh group. Moreover, there was no significant difference with regard to the ASC level between the SCI+Veh group and the SCI+A-68930 group.
Discussion
In the past years, A-68930, a DRD1 full agonist, is recognized as an efficient anti-stress agent.26, 27 Recent study has showed that A-68930 can exert protective effects on neurons through controlling 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation.20 In the present study, we demonstrated that A-68930 administration suppressed NLRP3 inflammasome activation with a decrease in IL-1β, IL-18 and TNF-α levels, inhibition of MPO activity, reduction in pulmonary edema and vascular permeability, and alleviation of lung tissue damage in a rat model of SCI-induced ALI.
IL-1β has a pivotal role in acute lung damage,28 which is involved in increasing other proinflammatory cytokines such as TNF-α, induction of endothelium damage and apoptosis, enhancing vascular endothelial and alveolar epithelial permeability, contributing to edema, finally leading to cell death and epithelial barrier dysfunction.29, 30 IL-1β can cause ALI through alphavbeta5 and alphavbeta6 integrin-dependent mechanisms.31 Block of IL-1 receptor can suppress inflammation and fibrosis of mesenchymal stem cells in lung injury,32 ameliorate hypoxemia in LPS/mechanical ventilation-induced-ALI33 and attenuate lung injury in gram-negative pneumonia.34 IL-18 is another important proinflammatory cytokine in ALI.35 Bleomycin-induced lung injury was markedly alleviated in IL-18Rα−/− and IL-18−/− mice.36 TNF-α can inhibit vasodilator-stimulated phosphoprotein expression and weaken alveolar–capillary barrier function in ALI.36 Targeting the TNF-α too can attenuate cardiopulmonary bypass-induced lung injury.37 Anti-TNF-α antibody can alleviate inflammatory lung injury induced by cardiopulmonary bypass in a rabbit model.38 Consistent with previous studies, we found that trauma to spinal cord causes an increase in proinflammatory cytokine levels in the BALF.2 However, A-68930 treatment markedly downregulated the protein expressions of IL-1β, IL-18 and TNF-α. Furthermore, a decrease in the TNF-α level may be related to reduced IL-1β production, and further investigation is required.
Neutrophil influx into the lung tissue with increasing oxidative and proteolytic enzymes levels can induce endothelial cell damage and overwhelming inflammation.39 The intensity of neutrophil influx is related to the severity of lung injury in acute respiratory distress syndrome patients.40 Targeting neutrophil exocytosis can alleviate ALI in a rat model of immune complex deposition.41 Consistent with previous studies,2 trauma to spinal cord caused an increase in neutrophil infiltration and activation in lung tissue. However, our study showed that the increase in MPO activity was markedly inhibited by A-68930 administration.
Importantly, the NLRP3 inflammasome is increasingly recognized as an important proinflammatory mediator regulating the maturation and release of IL-1β and IL-18.20 Most recently, the NLRP3 inflammasome has been reported to have an important role in paraquat-,9 LPS-33 and mechanical ventilation42-induced ALI, and targeting of the NLRP3 inflammasome can inhibit inflammation and alleviate pulmonary edema and tissue damage in LPS-induced ALI rats.17 Silencing of NLRP3 inflammasome inhibits ceramide-induced alveolar epithelial permeability and proinflammatory cytokine secretion.29 NLRP3 deletion can suppress pulmonary inflammation and epithelial cell apoptosis in hyperoxia-induced ALI mice.43 Moreover, inhibition of NLRP3 inflammasome attenuated histopathologic changes, MPO activity and protein concentrations in the BALF in burn-induced ALI.44 In this study, we found that SCI caused NLRP3 inflammasome activation in the lung tissue, and A-68930 administration significantly inhibited the inflammasome activation.
Conclusion
In conclusion, our experimental findings indicated that A-68930 exhibited a protective effect on SCI-induced ALI by the alleviations of inflammatory response with the inhibition NLRP3 inflammasome activation 72 h post injury. The present study indicated that A-68930 could be a potentially efficient therapeutic strategy for the treatment of SCI-induced ALI.
Data archiving
There were no data to deposit.
References
Bao F, Brown A, Dekaban GA, Omana V, Weaver LC . CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp Neurol 2011; 231: 272–283.
Liu J, Yi L, Xiang Z, Zhong J, Zhang H, Sun T . Resveratrol attenuates spinal cord injury-induced inflammatory damage in rat lungs. Int J Clin Exp Pathol 2015; 8: 1237–1246.
Amatachaya S, Pramodhyakul W, Wattanapan P, Eungpinichpong W . Ability of obstacle crossing is not associated with falls in independent ambulatory patients with spinal cord injury. Spinal Cord 2015; 53: 598–603.
Tollefsen E, Fondenes O . Respiratory complications associated with spinal cord injury. Tidsskr Nor Laegeforen 2012; 132: 1111–1114.
Veeravagu A, Jiang B, Rincon F, Maltenfort M, Jallo J, Ratliff JK . Acute respiratory distress syndrome and acute lung injury in patients with vertebral column fracture (s) and spinal cord injury: a nationwide inpatient sample study. Spinal Cord 2013; 51: 461–465.
Alabed S, de Heredia LL, Naidoo A, Belci M, Hughes RJ, Meagher TM . Incidence of pulmonary embolism after the first 3 months of spinal cord injury. Spinal Cord 2015; 53: 835–837.
Bao F, Omana V, Brown A, Weaver LC . The systemic inflammatory response after spinal cord injury in the rat is decreased by alpha4beta1 integrin blockade. J Neurotrauma 2012; 29: 1626–1637.
Chen T, Mou Y, Tan J, Wei L, Qiao Y, Wei T et al. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 2015; 25: 55–64.
Liu Z, Zhao H, Liu W, Li T, Wang Y, Zhao M . NLRP3 inflammasome activation is essential for paraquat-induced acute lung injury. Inflammation 2015; 38: 433–444.
Schroder K, Tschopp J . The inflammasomes. Cell 2010; 140: 821–832.
Wang W, Wang C, Ding XQ, Pan Y, Gu TT, Wang MX et al. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol 2013; 169: 1352–1371.
Davis BK, Wen H, Ting JP . The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735.
Martinon F, Mayor A, Tschopp J . The inflammasomes: guardians of the body. Annu Rev Immunol 2009; 27: 229–265.
Lamkanfi M, Dixit VM . Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 2012; 28: 137–161.
Liu SB, Mi WL, Wang YQ . Research progress on the NLRP3 inflammasome and its role in the central nervous system. Neurosci Bull 2013; 29: 779–787.
Zhang QY, Pan Y, Wang R, Kang LL, Xue QC, Wang XN et al. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J Nutr Biochem 2014; 25: 420–428.
Tianzhu Z, Shihai Y, Juan D . The effects of morin on lipopolysaccharide- induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation 2014; 37: 1976–1983.
Salmi P, Ahlenius S . Sedative effects of the dopamine D1 receptor agonist A 68930 on rat open-field behavior. Neuroreport 2000; 11: 1269–1272.
Rasheed N, Ahmad A, Al-Sheeha M, Alghasham A, Palit G . Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci Lett 2011; 504: 151–155.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015; 160: 62–73.
Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S . The immunoregulatory role of dopamine: an update. Brain Behav Immun 2010; 24: 525–528.
Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S . Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun 2008; 373: 286–291.
Young W . Spinal cord contusion models. Prog Brain Res 2002; 137: 231–255.
Gao J, Zhou L, Ge Y, Lin S, Du J . Effects of different resuscitation fluids on pulmonary expression of aquaporin1 and aquaporin5 in a rat model of uncontrolled hemorrhagic shock and infection. PLoS ONE 2013; 8: e64390.
Gan L, Zhong J, Zhang R, Sun T, Li Q, Chen X et al. The immediate intramedullary nailing surgery increased the mitochondrial DNA release that aggravated systemic inflammatory response and lung injury induced by elderly hip fracture. Mediators Inflamm 2015; 2015: 587378.
Rasheed N, Ahmad A, Singh N, Singh P, Mishra V, Banu N et al. Differential response of A68930 and sulpiride in stressinduced gastric ulcers in rats. Eur J Pharmacol 2010; 643: 121–128.
Rasheed N, Ahmad A, Pandey CP, Chaturvedi RK, Lohani M, Palit G . Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem Res 2010; 35: 22–32.
Yan YM, Li YD, Song XL, Liu M, Diao F, Wang Y et al. Therapeutic effects of inhaling aerosolized surfactant alone or with dexamethasone generated by a novel noninvasive apparatus on acute lung injury in rats. J Trauma Acute Care Surg 2012; 73: 1114–1120.
Kolliputi N, Galam L, Parthasarathy PT, Tipparaju SM, Lockey RF . NALP-3 inflammasome silencing attenuates ceramide-induced transepithelial permeability. J Cell Physiol 2012; 227: 3310–3316.
Wu J, Xu X, Li Y, Kou J, Huang F, Liu B et al. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol 2014; 745: 59–68.
Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res 2008; 102: 804–812.
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007; 104: 11002–11007.
Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J et al. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol 2014; 50: 270–280.
Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 2011; 183: 1380–1390.
Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H . Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol 2009; 41: 661–670.
Tang M, Tian Y, Li D, Lv J, Li Q, Kuang C et al. TNF-α mediated increase of HIF-1α inhibits VASP expression, which reduces alveolar-capillary barrier function during acute lung injury (ALI). PLoS ONE 2014; 9: e102967.
Gao M, Xie B, Gu C, Li H, Zhang F, Yu Y . Targeting the proinflammatory cytokine tumor necrosis factor-α to alleviate cardiopulmonary bypass-induced lung injury (review). Mol Med Rep 2015; 11: 2373–2378.
Yu Y, Gao M, Li H, Zhang F, Gu C . Pulmonary artery perfusion with anti-tumor necrosis factor alpha antibody reduces cardiopulmonary bypass-induced inflammatory lung injury in a rabbit model. PLoS ONE 2013; 8: e83236.
Abraham E . Neutrophils and acute lung injury. Crit Care Med 2003; 31: S195–S199.
Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M et al. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care 2015; 19: 53.
Uriarte SM, Rane MJ, Merchant ML, Jin S, Lentsch AB, Ward RA et al. Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock 2013; 39: 286–292.
Kuipers MT, Aslami H, Janczy JR, van der Sluijs KF, Vlaar AP, Wolthuis EK et al. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology 2012; 116: 1104–1115.
Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK et al. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol 2013; 305: C182–C189.
Han S, Cai W, Yang X, Jia Y, Zheng Z, Wang H et al. ROS-mediated NLRP3 inflammasome activity is essential for burn-induced acute lung injury. Mediators Inflamm 2015; 2015: 720457.
Acknowledgements
This study was supported by Traditional Chinese Medicine Science and Technology Project of Zhejiang (grant no.2011ZA078).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jiang, W., Li, M., He, F. et al. Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury. Spinal Cord 54, 951–956 (2016). https://doi.org/10.1038/sc.2016.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.52
This article is cited by
-
NLRP3 Inflammasome-Mediated Neuroinflammation and Related Mitochondrial Impairment in Parkinson’s Disease
Neuroscience Bulletin (2023)
-
Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship
Journal of Neuroinflammation (2016)