Abstract
Study design:
Case report.
Objectives:
To describe the case of a spinal cord injury patient that went scuba diving resulting in a mechanical deformation of his intrathecal baclofen pump.
Setting:
University Hospitals Leuven, Belgium.
Methods:
Case report.
Results:
Diving below 10 meters of depth can result in irreversible mechanical damage of the drug reservoir of an intrathecal baclofen pump.
Conclusion:
Patients with an intrathecal baclofen pump should be warned for the risks associated with scuba diving and should not dive more than 10 meters below sea level.
Similar content being viewed by others
Introduction
Intrathecal baclofen (ITB) is a safe method for treating spasticity that cannot be controlled by oral medication. Complications related to ITB therapy mostly consist of catheter-related problems.1, 2
People with spinal cord injury tend to be and are encouraged to be active even with an ITB pump. Often they take part in different sport activities.
Case presentation
A 37-year-old male with a T4 spinal cord injury paraplegia, American Spinal Injury Association (ASIA) Impairment Scale3 A, secondary to a road traffic accident in 2003 presents at the outpatient clinic in September 2012 for refill of his ITB pump (SynchroMed II, Medtronic, Minneapolis, MN, USA), which was placed at our centre in August 2008 for uncontrollable spasticity of the lower limbs. Spasticity is well controlled and the patient does not have any specific complaints.
During refill procedure, only 27 ml can be injected in the reservoir whereas the reservoir of the pump is made for 40 ml.
During anamnesis the patient tells us that 3 weeks before, the patient went scuba diving to a depth of 30 meters below sea level.
The drug reservoir contained about 16 ml of baclofen on the day the patient went diving. He does not recall any altering of spasticity during or after diving.
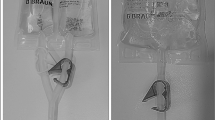
X-ray reveals a collapse of the bottom shield of the baclofen pump. (Figure 1).
This mechanical deformation is believed to cause irreversible loss of drug reservoir capacity.
Discussion
Searching PubMed, no literature concerning ITB pump dysfunctions during or after scuba diving could be found.
Scuba diving with an ITB pump is restricted by the company to a depth of 10 m or 33 feet below sea level.4 Testing performed by Medtronic has shown that the SynchroMed II pump may be damaged during a single exposure when the pump is not full and exposed to pressures >2.0 ATA (atmospheres absolute), or with repeated exposure to increased pressures even if they are <2.0 ATA.
Besides the permanent effect of a collapsed bottom shield, there is also the temporary effect of reduced flow rate. Flow rate accuracy decreases by ∼3% for a pressure of up to 2.5 ATA (15 m or 49 feet below sea level). At a pressure of 3.0 ATA, the SynchroMed II pump is not able to develop sufficient pressure to dispense and flow is stopped although the pumphead continues to rotate. The pump returns to normal function upon returning to local atmospheric pressure. Therefore we decided not to replace the baclofen pump in our symptom-free patient.
Conclusion
This is the first clinical case published of a patient with a mechanical deformation of an ITB pump following scuba diving.
We hope this case will alert doctors working with ITB pumps so that patients get sufficiently warned for the complications of diving with an ITB pump.
References
Penn RD, Kroin JS . Intrathecal baclofen alleviates spinal cord spasticity. Lancet 1984; 12: 1078.
Plassat R, Verbe BP, Menei P, Menegalli D, Mathé JF, Richard I . Treatment of spasticity with intrathecal baclofen administration: long-term follow-up, review of 40 patients. Spinal Cord 2004; 42: 686–693.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011; 34: 535–545.
Medtronic.com http://www.medtronic.com/patients/severe-spasticity/living-with/daily-living/activity-precautions/index.htm 2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Draulans, N., Roels, E., Kiekens, C. et al. Permanent mechanical deformation of an intrathecal baclofen pump secondary to scuba diving: a case report. Spinal Cord 51, 868–869 (2013). https://doi.org/10.1038/sc.2013.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.43