Abstract
Study design:
Spinal cord injury (SCI) results in gastrointestinal (GI) complications, including gastroesophageal reflux disease and constipation, but much of the data is based on older technology.
Objective:
GI transit times were determined in subjects with SCI using a new device called a SmartPill. Our principal goal was to assess whether this new technology can be applied in persons with SCI.
Methods:
SCI and age- and gender-matched able-bodied (AB) control subjects not taking proton pump inhibitors were studied. Following an 8-h overnight fast, subjects consumed 120 g EggBeaters (60 kcal), two slices of white bread (120 kcal) and 30 g strawberry jam (74 kcal). A pH calibrated SmartPill capsule was swallowed with 8 ounces of water, after which subjects fasted for an additional 6 h prior to consuming an Ensure Plus nutrition shake (350 kcal). Subjects remained fasted for an additional 2 h, after which time they resumed their regular diets.
Results:
Twenty subjects with SCI and 10 AB control subjects were studied. Data are expressed as mean±s.d. Comparing the group with SCI to the AB control group, gastric emptying time (GET), colonic transit time (CTT) and whole gut transit time (WGTT) were prolonged (GET: 10.6±7.2 vs 3.5±1.0 h, P<0.01; CTT: 52.3±42.9 vs 14.2±7.6 h, P=0.01; WGTT: 3.3±2.5 vs 1.0±0.7 days, P<0.01). No complications or side effects were reported.
Conclusion:
Our results indicate that the SmartPill technology is a safe, non-invasive assessment technique that provides valid diagnostic information in persons with SCI.
Similar content being viewed by others
Introduction
Gastrointestinal (GI) function is often assessed using manometry, radiopaque imaging and/or hydrogen breath testing.1, 2 These assessment techniques are invasive, use ionizing radiation, and often require patients to significantly change their normal lifestyle in preparation for evaluation. A relatively new device, the SmartPill, offers the ability to assess many GI variables, including temperature, pressure, pH, gastric emptying time (GET), colonic transit time (CTT) and whole gut transit time (WGTT), while minimizing the invasive nature of investigation. The SmartPill is a small wireless capsule that is ingested by the patient. Prior to use, each capsule is linked to a patient's file contained in software provided by the SmartPill Corporation. The software provides simple, step-by-step instructions to activate, calibrate and administer each capsule. Following a standard meal, the capsule, approximately the size of a large vitamin, is ingested by the patient with 8 ounces of water. Once ingested, values for pressure, temperature and pH are transmitted continuously from the capsule to a small receiver carried by the patient until the capsule is excreted during bowel evacuation. During monitoring, the patient is permitted to leave the office with the receiver and resume his normal lifestyle. Once the capsule is excreted, the receiver is returned to the office and the data obtained are downloaded to the Smartpill software. Following the download, information regarding gastric pH, segmental transit times and motility are expressed graphically and in a comprehensive, written report provided by the software. This information can then be used for diagnostic purposes.
In able-bodied (AB) persons, SmartPill technology has been validated in multiple studies utilizing radiopaque imaging for comparison, and is currently approved by the Federal Drug Administration as a diagnostic tool.3, 4 A study conducted by Kuo et al.3 evaluated the ability of SmartPill to discriminate between healthy patients and those with gastroparesis. In 87 healthy subjects and 61 gastroparetics, GET values from SmartPill correlated well with data obtained from a simultaneously administered radiolabeled meal. Diagnostic accuracy of SmartPill GET to the curve obtained from gastric emptying scintigraphy between gastroparetics and healthy subjects was 0.83. The study concluded that SmartPill correlated well with gastric emptying scintigraphy and accurately discriminates between healthy subjects and those with gastroparesis.3
The goal of this study was to evaluate the safety and efficacy of the SmartPill GI monitoring system as a diagnostic tool in persons with SCI.
Methods
Subjects
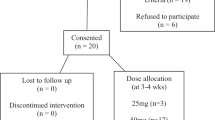
Individuals with complete or incomplete paraplegia (PARA) and tetraplegia (TETRA), who were injured for ⩾6 months, were eligible for recruitment for participation in the study. Subjects were required to have one or both of the following conditions: (1) routine bowel care lasting 30 min or greater or (2) episodes of fecal incontinence and/or constipation at least once per month. Age-matched AB control subjects with no history of GI pathology were included for comparison. Exclusion criteria included current treatment for gastroesophageal reflux disease, inflammatory bowel disease, diabetic polyneuropathy, previous abdominal or perianal surgery, peripheral nervous system prosthesis (for example sacral nerve stimulation), severe dysphagia, implanted electro-mechanical medical devices (cardiac pacemakers, infusion pumps) or known food allergies to any component of the standard test meal. Approval for this investigation was obtained from the Institutional Review Board at the James J Peters Veterans Affairs Medical Center, Bronx, New York, prior to initiating recruitment or study of subjects.
Procedures
Following an 8-h overnight fast, subjects consumed a standard test meal consisting of 120 g EggBeaters (equivalent to two large eggs (60 kcal)), two slices of white bread (120 kcal) and 30 g strawberry jam (74 kcal). A pH calibrated SmartPill capsule was swallowed with 8 ounces of water. Subjects were provided with a portable data receiver and remained fasted for 6 h following capsule ingestion, at which time they consumed an Ensure Plus nutrition shake (350 kcal). Subjects remained fasted for an additional 2 h, after which time they resumed their normal diets. Data were collected by the data receiver, which was carried by the subject until the capsule was excreted in a bowel movement.
GET was defined as the time elapsed between capsule ingestion and the first abrupt rise in pH (⩾3 units). CTT was defined as the time elapsed between the ileocecal junction (as indicated by an abrupt decrease in pH following the abrupt rise in pH after gastric emptying) and capsule exit from the body (evidenced by an abrupt decrease in temperature). WGTT was defined as the time elapsed between capsule ingestion and its expulsion from the body.
Statistical methods
Analysis of variance was performed for transit times, and maximum gastric acidity in PARA, TETRA and AB subjects with Fischer's protected least-significant difference post hoc test. The results are expressed as mean±s.d. The statistical tests were performed using StatView version 5.0 (SAS Institute, Cary, NC, USA). The harmonic mean for the SCI and AB control groups (13.3) was used to calculate power, because each group had an unequal number of subjects. The harmonic mean was calculated by dividing twice the product of the number of subjects in each group [2(10)(20)=400] by the total number of subjects (30).
Results
Subjects
There were 12 subjects with PARA (11 males, 1 female; 52±14 years) and 8 subjects with TETRA (7 males, 1 female; 41±9 years). The PARA group had a mean duration of injury (DOI) of 15±11 years, and the TETRA group had a DOI of 14±12 years. In the PARA group, eight subjects had complete motor/sensory injury and four subjects had incomplete motor/sensory injury; in the TETRA group, four had complete motor/sensory injury and four subjects had incomplete motor/sensory injuries (Table 1). There were no significant differences in distribution of injury (that is complete vs incomplete) or DOI between the PARA and TETRA groups. Ten AB subjects (eight males, two females; 40±12 years) were also studied, and there was no significant difference in age or distribution of gender, between the SCI and AB groups.
GET
GET was prolonged in the group with SCI compared with the AB group (10.6±7.2 vs 3.5±1.0 h, P<0.01, respectively; power=100% based on harmonic mean (n=13.3) for SCI vs AB; Figure 1). There was no significant difference between the PARA and TETRA groups (11.0±7.0 vs 10.1±7.9, P>0.7, respectively).
Maximum gastric acidity
Minimum values for gastric pH were similar in the group with SCI and the AB group (1.1±0. 8 vs 1.0±0.8 pH units, P>0.6, respectively; power <15% based on harmonic mean (n=13.3) for SCI vs AB). There was no significant difference between the PARA and TETRA groups. Maximum gastric acidity and DOI were not correlated.
CTT
CTT was prolonged in the group with SCI compared with the AB group (52.3±42.9 vs 14.2±7.6 h, P=0.01, respectively; power=100% based on harmonic mean (n=13.3) for SCI vs AB; Figure 1). There was no significant difference between the PARA and TETRA groups (58.2±47.2 vs 43.4±36.7 h, P=0.5, respectively). CTT and DOI were not correlated.
WGTT
WGTT was prolonged in the group with SCI compared with the AB group (3.3±2.5 vs 1.0±0.7 days, P<0.01, respectively; power=100% based on harmonic mean (n=13.3) for SCI vs AB). There was no significant difference between the PARA and TETRA groups (3.5±2.7 vs 3.0±2.3 days, P>0.6, respectively). WGTT and DOI were not correlated.
Discussion
Our data using the SmartPill indicate that transit times are prolonged in persons with SCI, which is consistent with previous findings using more invasive assessment techniques. As in the AB population, the present approach is arguably the most non-invasive method of determining GI transit times after SCI. Nevertheless, our results appear to confirm prior technology involving ingested radiopaque markers and indwelling manometry catheters.
Although the primary intent of this study was to assess the application of this technology to a unique subgroup of persons, our results, nonetheless, may have implications in terms of the pathogenesis of gastroesophageal reflux disease, constipation and fecal impactions after SCI. Gastroesophageal reflux disease has been associated with multiple potential causes, including delayed gastric emptying, lower esophageal sphincter dysfunction, lifestyle factors including obesity and smoking, supine or recumbent positioning, and excess gastric acidity.
This study has evaluated asymptomatic patients with SCI. Although gastric emptying of the SmartPill may not be an exact representation of physiologic gastric emptying of a mixed solid and liquid meal, given that all subjects followed the same testing procedures, we are clearly able to demonstrate that gastric emptying is, in fact, delayed in paraplegic and tetraplegic patients, relative to our control group. Similar findings using radiolabeled solid and liquid meals are found in the literature.5, 6 A study conducted by Zhang et al.7 found conflicting findings, suggesting that SCI does not affect gastric emptying. However, the consensus of the literature, including the present data, indicates that gastric emptying is, in fact, delayed in this population.
An increased incidence of constipation and impactions in patients with SCI is a well-known medical consequence of SCI. Despite extensive lifestyle modifications, medical interventions and regular, invasive bowel care techniques, many patients with SCI continue to have bowel dysfunction.8 It is known that decreased sacral parasympathetic innervations and colonic motility can result in prolonged CTT.9 Our data support previous findings (using less advanced technology) and demonstrate that CTT is delayed in both paraplegic and tetraplegic patients, with no significant difference between the two subgroups.10, 11 The colon is largely responsible for absorption of water from digestive waste, and prolonged colonic transit may result in hardened stool and subsequent constipation or impaction.
All subjects enrolled in this study successfully ingested and excreted the SmartPill capsule without side effects or complications, including esophageal or intestinal obstruction. All subjects with SCI, even those with high-level cervical lesions, were able to swallow the capsule without undue distress. All subjects reported that they would undergo the evaluation again in the future, if necessary, and no subjects reported complications or side effects of any kind.
Therefore, no adverse side effects were observed during this study. However, several factors should be taken into consideration prior to adopting this technology for clinical use. There is a high initial investment for the SmartPill software and each capsule is an additional expense.
In conclusion, SmartPill is a safe, valid approach and we can confidently support the use of this technology for future GI investigations in persons with SCI. Additionally, our SmartPill data seem to indicate that gastric emptying, colonic and cumulative WGTTs are prolonged in persons with SCI.
References
Verbeke K, de Preter V, Geboes K, Daems T, van den Mooter G, Evenepoel P et al. In vivo evaluation of a colonic delivery system using isotope techniques. Aliment Pharmacol Ther 2005; 21: 187–194.
Kasicka-Jonderko A, Kotula I, Sojka E, Syrkiewicz-Trepiak D, Jonderko K . [Application of the hydrogen breath test for the evaluation of oro-caecal transit time]. Wiad Lek 2003; 56: 172–179.
Kuo B, McCallum RW, Koch KL, Sitrin MD, Wo JM, Chey WD et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008; 27: 186–196.
Cassilly D, Kantor S, Knight LC, Maurer AH, Fisher RS, Semler J et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008; 20: 311–319.
Segal JL, Milne N, Brunnemann SR . Gastric emptying is impaired in patients with spinal cord injury. Am J Gastroenterol 1995; 90: 466–470.
Kao CH, Ho YJ, Changlai SP, Ding HJ . Gastric emptying in spinal cord injury patients. Dig Dis Sci 1999; 44: 1512–1515.
Zhang RL, Chayes Z, Korsten MA, Bauman WA . Gastric emptying rates to liquid or solid meals appear to be unaffected by spinal cord injury. Am J Gastroenterol 1994; 89: 1856–1858.
Singal AK, Rosman AS, Bauman WA, Korsten MA . Recent concepts in the management of bowel problems after spinal cord injury. Adv Med Sci 2006; 51: 15–22.
Fajardo NR, Pasiliao RV, Modeste-Duncan R, Creasey G, Bauman WA, Korsten MA . Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol 2003; 98: 128–134.
Menardo G, Bausano G, Corazziari E, Fazio A, Marangi A, Genta V et al. Large-bowel transit in paraplegic patients. Dis Colon Rectum 1987; 30: 924–928.
Leduc BE, Giasson M, Favreau-Ethier M, Lepage Y . Colonic transit time after spinal cord injury. J Spinal Cord Med 1997; 20: 416–421.
Acknowledgements
The Department of Veteran Affairs Rehabilitation Research and Development Service Center of Excellence for the Medical Consequences of Spinal Cord Injury (#B4162-C) and the James J Peters VA Medical Center have provided the support that made this work possible.
Guarantor of the article: Mark A Korsten, MD, is accepting full responsibility for the conduct of the study.
Specific author contributions: Robert E Williams III, MS, was responsible for the development of the study protocol, obtaining local human subjects committee approval, supervising and implementing the study. He was involved in data collection, analysis and interpretation. Mr Williams is the first author of this manuscript, and he has approved the final draft submitted for consideration for publication.
Dr William A Bauman assisted in writing the protocol and supported its performance. Dr Bauman was closely involved in writing and editing the manuscript, and he has approved the final draft that is being submitted for consideration for publication.
Dr Ann M Spungen assisted in the development and implementation of the protocol, data analysis and writing the manuscript. Dr Spungen has approved the final draft submitted for consideration for publication.
Dr Ravi R Vinnakota consented subjects, implemented study procedures and compiled final reports for each subject. Dr Vinnakota has approved the final draft submitted for consideration for publication.
Dr Rany Z Farid consented subjects, implemented study procedures and compiled final reports for each subject. Dr Farid has approved the final draft submitted for consideration for publication.
Dr Marinella Galea was responsible for identifying eligible subjects for study participation. Following completion of the study, she assisted in analysis and interpretation of the findings. Dr Galea has approved the final draft submitted for consideration for publication.
Dr Mark A Korsten was the primary investigator on the study protocol, and he is the Chief of the Gastroenterology Service at the James J Peters VA Medical Center. Dr Korsten was responsible for acquiring the SmartPill technology used in this study and supervised all study activities, including data collection, analysis and interpretation. Dr Korsten has approved the final draft submitted for consideration for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Williams, R., Bauman, W., Spungen, A. et al. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord 50, 81–84 (2012). https://doi.org/10.1038/sc.2011.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.92
Keywords
This article is cited by
-
The Wireless Motility Capsule: a One-Stop Shop for the Evaluation of GI Motility Disorders
Current Gastroenterology Reports (2016)
-
Towards an avatar mentor framework to support physical and psychosocial treatments
Health and Technology (2012)