Abstract
Study design:
Prospective repeated-measures longitudinal study.
Objectives:
To determine if an 8-week course of an oral anabolic steroid can positively effect body composition or pulmonary function in healthy individuals with chronic tetraplegia.
Setting:
United States.
Methods:
Oxandrolone (20 mg per day) was administered for 8 weeks to 10 men with motor complete tetraplegia. Dual X-ray absorptiometry scans, pulmonary function tests (PFTs), serum lipids and liver function tests (LFTs) were obtained at baseline, 4, 8, 12 and 20 weeks. To analyze change over time, a repeated measures General Linear Model and nonparametric tests were utilized.
Results:
Following treatment, total lean body mass (LBM) increased 1.9% and LBM of the arms increased 5.4%. Total body fat decreased 1.5%, and increased 3.9% in the arms and, on average, combined measures of PFTs improved 2.2%. High-density lipoprotein cholesterol decreased 31.8%, low density lipoprotein cholesterol increased 41.2%, and LFTs increased 9.7–65.6% while on therapy but all trended to baseline at 20 weeks.
Conclusion:
Baseline body composition was characterized by a high proportion of fat and a body mass index that underestimated chronic disease risk. Treatment with oxandrolone was associated with modest improvements in PFTs and in arm and total body LBM. Unfavorable changes in serum lipids and LFTs indicate that reported benefits of using oxandrolone in this population must be carefully weighed against potential adverse effects.
Similar content being viewed by others
Introduction
Individuals with acute spinal cord injury (SCI) undergo a catabolic period characterized by nitrogen wasting and loss of lean body mass (LBM).1 With time and the inactivity associated with SCI, significant changes in body composition occur with an increase in fat mass and a decrease in muscle mass.2, 3 The acute loss of muscle mass is one contributing factor to the profound alterations in body composition seen in long-term SCI. Another factor is the reduction in body cell mass of 34% in paraplegics and 49% in tetraplegics.4 Spungen et al.5 used dual energy X-ray absorptiometry (DXA) to assess body composition and found significant decreases in regional and total body lean tissue percentages in men with paraplegia and tetraplegia compared with controls. Based on this, individuals with tetraplegia can expect to lose approximately 3.0% of their total LBM per decade compared with 1% per decade in the able-bodied population.5
In addition to a loss of LBM, individuals with SCI incur some degree of pulmonary insufficiency due to muscle weakness directly related to the level of neurologic impairment.6, 7 Linn et al.6 quantified the loss of pulmonary function in subjects with SCI by level of injury compared with able-bodied controls. Forced vital capacity (FVC) was 45% of predicted for those with C4 SCI, 81% of predicted when the injury was at the T5 level and 95% of predicted for those with T12 SCI. This decreased pulmonary function results in pulmonary complications that are the most common cause of death and the second leading cause of morbidity in persons with long-term SCI.8, 9
Anabolic steroids have been shown to reduce nitrogen wasting in persons with HIV10 and severe burns,11 and be beneficial in individuals with chronic obstructive disease.12 Preliminary data using oxandrolone suggest that anabolic steroids might also be beneficial in reducing or minimizing some of the complications following SCI.13, 14 A retrospective case series described 9 individuals with SCI who had nonhealing pressure ulcers. After being treated with oxandrolone for 1–12 months (20 mg per day) and glutamine, 8 of 9 patients had healed wounds.13 In a small pilot study examining pulmonary function after a 1-month course of oxandrolone (20 mg per day), individuals with chronic SCI gained an average of 1.4 kg, had improved combined spirometry measures of 9% and reported less dyspnea.14
The purpose of this pilot study is to expand on this experience with oxandrolone and describe the effect of an 8-week course of 20 mg per day on body composition and pulmonary function in men with tetraplegia.
Materials and methods
Subjects
Men with SCI were recruited from the outpatient clinic population at the National Rehabilitation Hospital, Washington, DC using fliers and word of mouth. Eligible men were between the ages of 18 and 65, at least 1 year post-injury, had a motor complete injury between C4 and C8 (American Spinal Injury Association Impairment Scale (AIS) A or B),15 had access to reliable transportation and could be readily reached by telephone. Individuals were excluded who had an active pulmonary infection or pressure ulcer, a history of liver disease, heart disease, diabetes mellitus, or were taking warfarin, dantrolene sodium, carbamazepine or lipid-lowering agents. Institutional review board (IRB) approval was obtained from the MedStar IRB and all subjects provided informed consent before enrolling in the study.
Design
This was a prospective repeated-measures pilot study of 10 subjects who took 20 mg oxandrolone orally every day in divided doses for 8 weeks. Each individual served as his own control with the baseline session providing the control measurement. Subjects were then followed for an additional 12 weeks after the treatment period. During this 20-week study period, they were seen in the clinic on 5 occasions as follows: weeks 1 (baseline), 4, 8, 12 and 20. Before taking the first dose of oxandrolone, the following baseline measurements were obtained: height and body weight, urinalysis, complete blood count, metabolic panel, lipid profile, DXA and pulmonary function tests (PFTs). Height was measured while subjects were recumbent in the DXA scanner and body weight was obtained using a Hoyer Lift Scale. DXA measurements were made with a Lunar DXA Instrument using Prodigy Software (GE Medical System, Pittsburg, PA, USA). Any metal objects that were worn or in clothing were removed before the scans. DXA examinations provided measures of body composition for LBM and body fat (BF) for the total body and upper extremities.
The metabolic panel included the following liver function tests (LFTs): serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactic dehydrogenase (LDH) and albumin (ALB). The lipid profile included total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TRIG) and calculated low-density lipoprotein cholesterol (LDL-C). Except for height and urinalysis, these tests were repeated at each of the subsequent four visits.
Body mass index (BMI) was calculated by dividing the weight (kg) by the height (m2). Subjects were stratified by BMI according to the following: Underweight (<18.5 kg m−2), Normal (18.5–24.9 kg m−2), Overweight (25.0–29.9 kg m−2) or Obesity Class I (30.0–34.9 kg m−2), Obesity Class II (35.0–39.9), or Obesity Class III (⩾40.0).16
Pulmonary function measurements were obtained with subjects seated in their wheelchairs using open-circuit spirometry equipment (Model TrueOne 2400; Parvo Medics, Sandy, UT, USA). A minimum of three trials were performed according to the recommendations of the American Thoracic Society.17 The values for FVC, forced expiratory volume in one second (FEV1), the ratio of FEV1/FVC, peak expiratory flow rate (PEFR) and maximum ventilator volume (MVV) were obtained and the best efforts for FVC, FEV1 and MVV were recorded. Spirometry results were reported as absolute values and compared to standards for tetraplegic individuals.18
Subjects were called once weekly at home to monitor drug compliance, health status and possible adverse drug effects. An interval health history with particular attention to new medical problems and potential medication side effects and a comprehensive physical exam were performed at each of the five clinic visits.
Statistical analyses
Statistical analyses based on the General Linear Model, nonparametric Friedman Rank ANOVA and Estimated Marginal Means were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) for Windows. Repeated measures methods were used to analyze within-subject variance associated with change in study variables from baseline to posttreatment. To account for a Type I error, a P value was established at 0.05.
Results
All subjects had tetraplegia with the following distribution: C5 (2), C6 (4), C7 (3) and C8 (1). The neurological levels of seven participants were AIS A and of three were AIS B.15 The mean age was 32.5 years (range: 23–50) and the average duration of injury was 8.8 years (range: 2–26). The average weight was 78.8 kg (range: 46.7–105.6) and the mean height was 1.8 meters (range: 1.7–1.9).
The mean BMI at baseline was 23.8 (range 12.8–31.2), corresponding to a mean BF% of 33.7% (range 17.1–43.4%). When stratified by baseline BMI, two subjects were in the Underweight category and three were in the Normal category whereas four individuals were classified as Overweight and one was in the Obese category (Table 1). There were four subjects with BF greater than 40% who's BMI were classified in the Normal, Overweight or Obese categories.
Mean baseline values for total body LBM and BF were 48.7 and 26.7 kg, respectively, whereas similar values for LBM and BF for the arms were 5.6 and 2.6 kg, respectively (Table 2). Total body composition showed a significant mean percent increase in LBM of 1.9 (P=0.02) after 8 weeks of therapy and a mean percent decrease in BF of −1.5 that did not reach significance (P=0.35). In the arms, there was an average increase in LBM of 5.4% (P=0.51) and in BF of 3.9% (P=0.43) while on oxandrolone. Twelve weeks following the course of oxandrolone (study week 20), the mean percent of LBM returned to just below baseline or back to baseline for total body and arms whereas mean BF for total body increased by 2.6% (P=0.42), and decreased by −15.4% (P=0.37) for arms (Table 2).
For PFTs, baseline mean FVC was 2.76l (±0.89), FEV1 was 1.94 l (±0.71), FEV1/FVC was 3.81% (±2.81), PEFR was 3.81 l (±2.58) and MVV was 82.19 l (±23.60). Mean percent changes in PFTs following treatment increased for FVC, FEV1 and MVV (3.3, 3.1 and 9.3, respectively) and decreased by 3.4% for PEFR but none of these changes reached significance. At the 20-week follow-up, mean FVC was 2.81 l (±0.60), FEV1 was 1.99l (±0.42), FEV1/FVC was 70.82%, PEFR was 3.28l (±1.57) and MVV was 85.89 l (±16.66), with none of these changes being statistically significant.
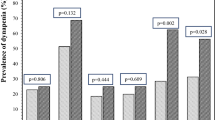
The effects of an 8-week course of oxandrolone on lipid and LFT values are presented in Figure 1. The lipid profile and LFTs were normal in all subjects at baseline. These tests became abnormal or trended toward abnormal during oxandrolone treatment with the exception of TRIG and ALB, which changed on average less than 1%. The mean values for LDL-C and TC increased from 83.9 to 118.5 mg per 100 ml and 147.2 mg per 100 ml to 169.2 mg per 100 ml, respectively, and for HDL-C decreased from 41.8 to 28.5 mg per 100 ml while on therapy, with all three values reaching a maximum change at week 4. The largest change for LDL-C was 41.2% (P=0.028) and for TC was 14.9% (P=0.015) whereas the maximum decline for HDL-C was −31.8% (P=0.002). The mean values for ALT, AST and LDH increased by 65.5, 44.5 and 9.7%, respectively, during week 8 of the treatment. Changes in ALT, AST and LDH were not statistically significant. By week 20 all of the lipid tests and LFTs returned to baseline or the normal range.
(a) Lipid profile. Chol, cholesterol; TRIG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol. (b) Liver function tests: ALT, AST and ALB. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin. (c) Liver function test: LDH. LDH, lactic dehydrogenase.
Normal laboratory values for lipids and LFTs are: TC 100–200 mg per 100 ml, TRIG 10–149 mg per 100 ml, LDL-C 0–99 mg per 100 ml, HDL-C 40–85 mg per 100 ml, ALT 13–51 IU/l, AST 10–42 IU/l, LDH 313–618 IU/l and ALB 3.5–5.0 mg per 100 ml.
Discussion
Preliminary data using the anabolic steroid, oxandrolone, for 4 weeks suggests it might be beneficial in reducing or minimizing some of the complications following SCI. This study examined the effects of an 8-week course of oxandrolone on body composition, pulmonary function, serum lipids and LFTs in people with tetraplegia.
This group of healthy men with chronic tetraplegia had a mean baseline BF percentage of 33.7% (range: 17.1–43.4%). This is equivalent to a mean BMI of 23.8 kg m−2, and range of 12.8–31.2 kg m−2. As the BMI is an easy and quick number to calculate, it has become a valuable and widely used yardstick for classifying individuals with respect to obesity and related risk factors for morbidity and mortality. In 1998, the World Health Organization defined obesity in men as >22% BF.16 However, as this study and others have shown, the BMI has significant limitations when used to estimate disease risk due to overweight and obesity in populations with SCI, as it may underestimate fat mass due to altered body composition.19 Of the subjects described in this study, 9 out of 10 had BF greater than 22% and 4 out of 10 had BF greater than 40% (Table 1). Further, the five subjects who had BMIs in the Below Normal and Normal categories at baseline actually had a mean total BF of 26.5%.
An analysis of the changes in body composition, especially when compared with able-bodied populations, provides additional insights into the long-term effects of SCI. Table 3 summarizes data for regional and total body composition from our study, compared with a second group of tetraplegics and a control population of veterans reported by Spungen et al.5 The data for the two groups of tetraplegics are especially revealing when the percent of BF and LBM are viewed side-by-side with the non-SCI control group. For the total body, the subjects we studied had 142.3% BF and only 82.7% LBM compared to the controls. The disparities between those with SCI and controls persist in the arms, with BF and LBM at 143.6 and 78.5% of that observed in controls, respectively. Spungen et al.5 reported similar changes in body composition in a tetraplegic population which had a marked increase in percent BF and a sizeable decrease in percent LBM in the arms and the total body. Analogous body composition abnormalities have been reported in other groups of tetraplegics, reemphasizing the profound alteration in body composition as a result of SCI.20, 21
The reason for these shifts in body composition have been well documented.19, 21, 22 Following SCI, LBM begins to atrophy below the level of injury and is replaced by BF.23 This disproportionate amount of fat in individuals with SCI has serious implications for long-term morbidity and mortality.19 In particular, there is a growing body of literature that demonstrates the secretory products of adipocytes result in a cascade of events associated with dyslipidemia, hypertension and insulin resistance.19, 23, 24 According to the 2005 Consensus Panel of the International Diabetes Foundation, any two of these conditions plus obesity constitute the metabolic syndrome,25 reemphasizing the importance of having a better tool to estimate disease risk due to overweight and obesity than the current BMI calculation utilized in people with SCI.
Despite the presence of a high proportion of BF in our subjects, baseline lipids were normal. It is important to note, however, the extent of adverse effects on lipid metabolism resulting from this 8-week course of oxandrolone, which have not been previously published in detail in the SCI population. During and after treatment with oxandrolone, LDL-C, HDL-C and TC levels became significantly abnormal with LDL-C and TC peaking and HDL-C reaching a nadir at week 4. Both LDL-C and HDL-C remained abnormal through week 12 and then returned to the normal range by week 20. These lipid changes are particularly significant in light of the body composition changes noted above that place individuals with SCI at greater risk for metabolic and cardiovascular disease.
When the effect of a course of oxandrolone on liver function was examined, LFTs increased during treatment, but levels returned to baseline at week 20. Mean LFT changes were greatest for ALT and AST (increasing 65.6 and 44.5%, respectively). These results are similar to a report by Spungen et al.,14 who described a significant increase in ALT and a significant decrease in HDL-C in 10 subjects with tetraplegia after treatment with oxandrolone for 4 weeks. Other LFTs and serum lipids increased only slightly from baseline. Time for the abnormal values to return to normal was not described. In our series, abnormal serum lipids and LFTs returned to baseline or close to baseline and well within the normal range 12 weeks after oxandrolone was stopped (study week 20). Whether long-term use of oxandrolone might result in more pronounced abnormalities or underlying pathologic tissue changes needs to be explored further if oxandrolone is to be used as a therapeutic agent in the clinical setting.
PFTs at baseline were on average 82.6% of the reference values reported for other tetraplegics.18 Although we did observe an increase in three of four PFT values, none of the changes were statistically significant. This is in contrast to the study by Spungen et al.14 which found a statistically significant improvement in FVC, FEV1 and forced inspiratory vital capacity in a group of 10 subjects with tetraplegia treated with oxandrolone 20 mg per day for 1 month. The reasons for the discrepancy in PFTs between these two studies are unclear, though differing demographics (mean participant age, duration of injury and baseline BMI) and treatment duration (4 versus 8 weeks) may have played a role.
We conclude that treatment with the anabolic agent oxandrolone (20 mg per day) for 8 weeks may provide short-term limited positive effects in subjects with tetraplegia that are associated with modest improvement in PFTs and in arm and total body LBM. However, abnormal changes in serum lipids and LFTs during treatment with oxandrolone indicate that reported benefits of using anabolic steroids in the tetraplegic population do not appear to outweigh their adverse effects. Selected limited use, such as to promote wound healing, may prove to carry a more favorable risk/benefit profile.
References
Guttman L . Spinal Cord Injuries: Comprehensive Management and Research, 2nd edn. Blackwell Scientific Publications: London, 1976.
Castro MJ, Apple Jr DF, Staron RS, Campos GE, Dudley GA . Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol 1999; 86: 350–358.
Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P . Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 1995; 33: 674–677.
Cardus D, McTAggart WG . Body composition in spinal cord injury. Arch Phys Med Rehabil 1985; 66: 257–259.
Spungen AM, Adkins RH, Stewart CA . Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003; 95: 2398–2407.
Linn WS, Spungen AM, Gong Jr H, Adkins RH, Bauman WA, Waters RL . Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord 2001; 39: 263–268.
Baydur A, Adkins RH, Milic-Emili J . Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol 2001; 90: 405–411.
Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 1998; 36: 266–274.
McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ . Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999; 80: 1402–1410.
Abrams D . Use of androgens in patients who have HIV/AIDS: what we know about the effect of androgens on wasting and lipodystrophy. AIDS Read 2001; 11: 149–156.
Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN . The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007; 246: 351–360.
Sharma S, Arneja A, McLean L, Duerksen D, Leslie W, Sciberras D et al. Anabolic steroids in COPD: a review and preliminary results of a randomized trial. Chron Respir Dis 2008; 5: 169–176.
Spungen AM, Koehler KM, Modeste-Duncan R, Rasul M, Cytryn AS, Bauman WA . 9 clinical cases of nonhealing pressure ulcers in patients with spinal cord injury treated with an anabolic agent: a therapeutic trial. Adv Skin Wound Care 2001; 14: 139–144.
Spungen AM, Grimm DR, Strakhan M, Pizzicato PM, Bauman WA . Treatment with an anabolic agent is associated with improvement in respiratory function in persons with tetraplegia: a pilot study. Mt Sinai J Med 1999; 66: 201–205.
American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, Revised 2002. American Spinal Injury Association: Atlanta, 2002.
National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes for Health: Bethesda, MD, 1998. NIH publication No. 98-4083.
American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995; 152: 1107–1136.
Pithon KR, Martins LEB, Renno ACM, Abreu DC, Cliquet Jr A . Pulmonary function testing in quadriplegic subjects. Spinal Cord 2008; 46: 275–277.
Gater DR . Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007; 18: 333–351.
Rajan S, McNeely MJ, Warms C, Goldstein B . Clinical assessment and management of obesity in individuals with spinal cord injury. J Spinal Cord Med 2008; 31: 361–372.
Jones LM, Legge M, Goulding A . Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil 2003; 84: 1068–1071.
Buchholz AC, McGillivrary CF, Pencharz PB . Differences in resting metabolic rate between paraplegic able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003; 77: 371–378.
Gater D, Clasey J . Body composition assessment in spinal cord injury clinical trials. Top Spinal Cord Inj Rehabil 2006; 11: 36–49.
Olda E . The metabolic syndrome as a concept of adipose tissue disease. Hypertens Res 2008; 31: 1283–1291. Review.
Holt RI . International Diabetes Federation re-defines the metabolic syndrome. Diabetes Obes Metab 2005; 7: 618–620.
Acknowledgements
We are grateful to Margot Giannetti and Miriam I Spungen for their assistance in the preparation of the article. This project was funded, in part, by a grant from the Department of Defense Award No. DAMP-17-02-2-0032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 34th Annual Meeting of the American Spinal Injury Association, San Diego, CA, USA
Rights and permissions
About this article
Cite this article
Halstead, L., Groah, S., Libin, A. et al. The effects of an anabolic agent on body composition and pulmonary function in tetraplegia: a pilot study. Spinal Cord 48, 55–59 (2010). https://doi.org/10.1038/sc.2009.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.82