Abstract
Body composition and muscle strength are emerging aspects in idiopathic pulmonary fibrosis (IPF) clinical assessment. We aimed to study the relationships of handgrip strength (HGS) with anthropometric variables, body composition, and disease staging, and to evaluate the prevalence of dynapenia in 102 clinically stable IPF patients (70 M; mean age: 69.4 years). Fat-free mass (FFM), skeletal muscle (SM) were estimated with bioimpedance analysis. HGS was measured with a digital handle dynamometer for both dominant and non-dominant body sides. Dynapenia was identified according to six recognized criteria sets. Mean body mass index (BMI) was 28.2 ± 4.7 kg/m2, with a prevalence of overweight (BMI > 25 and < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2) of 35% and 37%, respectively. FFM and SM were greater in males, whereas percentage body fat was higher in women. HGS was higher and declined with age slightly more rapidly in men, showing a stronger correlation with FFM and SM. Dynapenia prevalence ranged from 20.6 to 56.9%, depending on the criteria used, and was more frequent in older patients and advanced disease. Dynapenia is highly prevalent in IPF. HGS is a promising proxy marker of muscle function to be used in clinical evaluation and follow-up programs.
Similar content being viewed by others
Introduction
The evaluation of nutritional status is an increasingly important area in the care process of patients with various chronic diseases. Concerning lung diseases, a consistent body of evidence has shown that in patients with chronic obstructive pulmonary disease (COPD) alterations in body composition and impaired muscle function negatively affect pulmonary function, comorbidities, hospitalization, mortality, etc1,2.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and poor-prognosis interstitial lung disease (ILD) of unknown cause whose incidence has steadily increased, varying from 2.8 to 19 cases per 100 ,000 people/year in Europe and North America, respectively3,4. Disease behavior is highly variable, with associated comorbidities having a significant prognostic impact5. Although IPF remains refractory to treatment, the current availability of anti-fibrotic drugs, i.e., nintedanib and pirfenidone, has contributed to a certain extent to increase life expectancy and improve quality of life by reducing lung function decline over time, as well as the rate of hospitalization and that of acute exacerbations6.
According to a recent expert consensus, only a few papers have provided information on the nutritional status of patients with IPF7. Of note, body weight loss was found to be an independent predictive factor of reduced survival7,8 with a low body mass index (BMI) in the 36 months before the diagnosis associated with increased mortality9. On the other hand, a previous large retrospective study found better survival in patients with BMI > 30 kg/m2 compared to those with a lower BMI10. Looking at body composition, a recent study estimated that about one-third of patients with IPF were malnourished, exhibiting a low fat-free mass (FFM)11. Likewise, a high FFM was predictive of better survival12, while a worse prognosis was associated with a reduced cross-sectional area of the erector-spinal muscle13.
As far as muscle function is concerned, dynapenia (low muscle strength), which is actually a relevant diagnosis in the clinical setting, is defined as the loss of muscle strength associated with aging (or even nutrition-related diseases) not caused by neurological or muscular disorders14. In this regard, it is worth recalling that low handgrip strength (HGS), a proxy marker of muscle strength and a major component of sarcopenia15, has been related in the elderly to all-cause and disease-specific mortality, future function, bone mineral density, fractures, cognition and depression, and problems associated with hospitalization16.
While muscle strength is frequently considered for prognosis purposes in COPD17, only a few studies have evaluated HGS in IPF or fibrotic ILD patients18,19,20, with no information on dynapenia, i.e., the age- and/or disease-associated marked loss of muscle strength not caused by neurologic or muscular abnormalities14. Kozu et al.20 have shown that there was a highly significant inverse correlation between HGS and the degree of dyspnea, while Guler et al.18 reported that in fibrotic interstitial lung disease muscle strength was inversely related to age and directly to weight. Very recently, low HGS has been observed on a preliminary basis (in comparison to predicted values) in a small group of patients with IPF21. The reduction of the skeletal muscle mass, evaluated by computed tomography (CT), has been associated to HGS, physical performance, dyspnea and survival in IPF patients22,23,24. Finally, reduced physical activity was related to disease severity17 and worse prognosis as well25,26.
Based on this background, since 2019 we included the assessment of nutritional status, body composition, and muscle strength in the routine clinical evaluation of patients with IPF. Our cross-sectional study was carried out in such patients to analyze in a real-life setting: (a) the variability of HGS and its determinants; (b) the prevalence of dynapenia using different diagnostic criteria, and (c) the relationship of HGS and dynapenia with anthropometric variables, body composition, lung function, disease severity, and anti-fibrotic therapies.
Results
The main demographic and clinical characteristics of the study population are reported in Table 1. There were no differences between male and female patients in terms of age. Males were heavier (+ 16.7%) and taller (+ 9.7%), while the mean BMI was slightly higher in females (+ 3.2%). Prevalence of underweight patients (BMI < 21 kg/m2) was 7.8%, while overweight (BMI > 25 and < 30 kg/m2) and obese patients (BMI ≥ 30 kg/m2) were 35.3% and 37.3% of the whole study group, respectively. The 70% and 5% of patients were former or current smokers, respectively, with a median value of 25 packs/year smoked (IR 10–48). Systemic arterial hypertension (54%), gastro-esophageal reflux (27%), and type II diabetes (22%) were the most prevalent comorbidities in the group as a whole with no gender differences. Ischemic cardiovascular disease was more prevalent in males (23 vs. 6%, p < 0.05) and thyroid disease in females (21 vs. 3%, p < 0.01), whereas pulmonary hypertension (14% of cases) was only slightly more frequent in males (16 vs. 9%; p = NS). The median disease duration was 14 months (IR 6–32), with most patients under anti-fibrotic treatment with either pirfenidone (n = 44) or nintedanib (n = 49). As shown in Table 1, most patients were in GAP stages II and III (mild to moderate disease) and in TORVAN stage III, in both cases with no significant gender differences.
Lung function parameters are reported for all patients and by gender in Table 2. A mild to moderate restrictive ventilatory pattern with a similar single-breath diffusing lung capacity of the carbon monoxide (DLCOsb) deficit was detected with no clinically significant differences between males and females. The six-minute walk distance was available in 92 patients with an overall median distance walked of 363 m (IR 233–528). Male patients (63) walked more meters than females (29), with median values of 424 m (IR 264–528) for men versus 330 m (IR 189–461) for women.
Body composition
Mean values of FFM (+ 39.6%), FFM index (FFMI) (+ 15.8%), skeletal muscle (SM) (+ 64.7%) and SM index (SMI) (+ 38.4%) were all greater in male patients (Table 3), while percentage body fat was higher in females (+ 41.6%). All these variables did not significantly vary by the GAP or TORVAN stage. Significant low values of FFMI and SMI were observed in 8.8% and 6.8% of the sample. Finally, body composition was not related to any lung function parameter and no differences emerged by comparing patients treated with nintedanib to those taking pirfenidone.
Handgrip strength
Table 3 shows that mean values of maximum-HGS (+ 71.8%), dominant (D)-HGS (+ 71.7%), and non-dominant (ND)-HGS (+ 72.1%) were greater in male compared to female patients with IPF (p < 0.001). In both genders, D side values were found to be higher compared to those of the ND side. Maximum-HGS declined with age slightly more rapidly in men (− 0.50 kg/year) than women (− 0.41 kg/year), being 30.2 ± 9.8 kg versus 26.4 ± 8.8 kg in patients aged < 75 years and ≥ 75 years, respectively (p < 0.001). The differences between genders and age groups persisted even after controlling weight, BMI, or body composition (data not shown).
The associations of HGS with anthropometric variables and body composition are summarized in Table 4. Maximum-HGS, as well as D-HGS and ND-HGS, directly correlated with weight and BMI and more strongly with FFM and SM. As far as pulmonary function is concerned, maximum-HGS correlated with total lung capacity (r = 0.355, p < 0.001), but not with the forced vital capacity (FVC), the DLCOsb and the 6 min walk test (6MWT) distance. Also, neither the dyspnea nor the muscular fatigue, estimated with the Borg scale at the beginning and at the end of the 6MWT, correlated with the HGS (data not shown).
Multiple regression analysis showed that combining gender (beta = 0.588), age (beta = 0.343), and weight (beta = 0.269) accounted for 67.0% of the variance in maximum-HGS (SEE = 5.53 kg; p < 0.001) whereas stature and BMI were not recognized as significant predictors. With respect to body composition, SM (beta = 0.462) emerged as the most important predictor along with gender (beta = 0.293) and age (beta = − 0.318), with adjusted R = 0.660 (SEE = 5.61 kg; p < 0.001).
When compared to the other patients with IPF, maximum-HGS, expressed as mean ± standard error (SE), was lower in underweight patients (24.8 ± 3.0 kg vs. 29.5 ± 1.0 kg, p < 0.05) and also in those with low FFMI (23.5 ± 3.0 kg vs. 29.7 ± 1.0 kg, p < 0.05). Furthermore, after adjusting for gender, maximum-HGS (mean ± SE) was higher in patients in the GAP I stage compared to the other ones (30.8 ± 1.1 vs. 27.9 ± 1.0 kg, p < 0.05), and the same was true when TORVAN I was compared to TORVAN II-III stages (32.3 ± 1.4 vs. 28.2 ± 0.8 kg, p < 0.05); these differences disappeared when data were adjused also for age. On the contrary, no differences came out by comparing patients treated with nintedanib to those taking pirfenidone.
Dynapenia
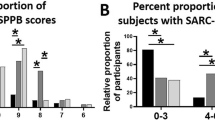
Six different criteria sets were used for identifying the dynapenic patients. The prevalence of dynapenia was higher using the TESSIER (56.9%), LAURETANI (39.2%), or FRIED (39.2%) criteria, compared to 23.5% with the EWGSOP-2, 20.6% with the ALLEY-1, and 21.6% with the ALLEY-2 criteria (Fig. 1). The percentage of dynapenic patients did not differ between genders with the EWGSOP-2 (22.9% vs. 25.0%), TESSIER (51.4% vs. 68.8%), ALLEY-1 (18.6% vs. 25.0%) or ALLEY-2 criteria (20.0% vs. 25.0%), but was higher in females than males using the LAURETANI (28.6% vs. 62.5%, p < 0.05) or FRIED (31.4% vs. 56.3%, p < 0.05) criteria.
A higher prevalence of dynapenia was observed in the patients with low BMI only using the TESSIER criteria. Those with low FFMI only used the TESSIER or LAURETANI criteria (data not shown). On the contrary, in all cases (except ALLEY-2) dynapenia was much more prevalent in IPF patients aged ≥ 75 years (Fig. 2), for instance, 46.4 versus 15.9% with the EWGSOP-2 criteria and 78.6 versus 48.6% with the TESSIER criteria.
The point prevalence estimate of dynapenia was greater (p < 0.05) in GAP stages II-III than GAP stage I with the EWGSOP-2 (32.7 vs. 13.0%), TESSIER (63.6 vs. 47.8%) and ALLEY-1 (27.3 vs. 13.0%) criteria, and in TORVAN stages II-III than TORVAN stage I with the EWGSOP-2 (27.6 vs. 12,0%), LAURETANI (43.4 vs. 24.0%), FRIED (43.4 vs. 24.0%), ALLEY-1 (26.3 vs. 4.0%) and ALLEY-2 (26.3 vs. 4.0%) criteria. Finally, no differences emerged in the prevalence of dynapenia by comparing patients treated with nintedanib to those taking pirfenidone, regardless of the small percentage of cases with mild GI side-effects (14%). Also, no patients reported > 5% weight loss in the three months preceding the study visit.
Discussion
To the best of our knowledge this is the first report addressing from a clinical point of view the prevalence distribution of dynapenia (i.e., low muscle strength) in IPF patients. It systematically evaluated HGS in this target population, showing lower values in underweight patients or with low FFM. Dynapenia was highly prevalent, even more in patients aged > 75 years. However, the estimated proportion varied depending on the criteria set used.
IPF is a chronic, progressive interstitial lung disease of unknown etiology3,18, characterized by a gradual decline of respiratory function up to death. Management and therapy of patients are still complex and not fully defined27,28. Disease progression may widely vary among patients: some rapidly deteriorate soon after diagnosis, others maintain a stable condition for years, and still, others show rapid decline following a period of stability29.
Alterations of nutritional status and/or body composition, which may negatively affect skeletal muscles, have seldom been described among the systemic manifestations of IPF30. To date, BMI has been evaluated in IPF patients with preliminary and sometimes contradictory results7; the findings of this study are consistent with previous data from European countries and the United States indicating that the mean value for BMI in patients with IPF was > 25 kg/m27. In effect, most of the patients studied were overweight or obese (no one with a BMI > 40 kg/m2), while the underweight ones were only 7.8% of the whole study sample. On the contrary, the estimated prevalence of low FFMI, which was around 8%, was less than reported in a recent paper by Jouneau et al.11; those authors did not give information about the bioimpedance analysis (BIA) equation used to predict FFM, whereas in the present study a well-validated equation for COPD patients was chosen31.
As partly surprising and additional information, there was no relationship between FFM and SM with clinical staging systems such as GAP or TORVAN.
Although it is well known that muscle function is related to morbidity, mortality, hospitalization, quality of life, etc. in the elderly and various diseases, i.e., COPD16,17, only a few studies have evaluated HGS in IPF patients18,19,20, showing significant correlations of HGS with age (inverse) and weight (direct)18. Besides, mean HGS value was found to be lower in more severe patients19,20. Very recently, low HGS has been observed on a preliminary basis (in comparison to predicted values) in a small group of patients with IPF21. Of note, HGS values largely varied between studies, while the association with body composition has never been taken into consideration, nor dynapenia has been diagnosed. Unfortunately, we do not have direct information of dynapenia in our local population. Indeed, it is interesting to compare our results on HGS with those given in the very relevant paper by Dodds et al.32 regarding normative values for grip strength in the general population. Twenty-eight per cent of male patients and 25% of female patients with IPF had HGS below the 10th percentiles (for age and gender) reported by Dodds et al. and 53% and 63% below the 25th percentiles, thus strongly suggesting low muscle strength. Interestingly, we have derived percentiles for HGS in our local population for adults aged 20–60 years that are very similar to the Dodds ones. In addition, the prevalence of dynapenia in IPF patients is similar to that we have observed in COPD (unpublished results), a chronic lung disease characterized by well-known impairments in the musculoskeletal system.
The findings of our study make it possible to assess the relationships of HGS with various potential predictors. In line with that has been observed in the general population32 and COPD patients33 HGS showed an inverse correlation with age, as already reported18, a direct correlation with weight, FM, and (slightly stronger) with FFM and SM. These observations were confirmed by multiple regression analysis. Models including gender, age explained around 70% of the total variance, and weight or (as an alternative) gender, age and FFM; in other words, no increase in the prediction power was observed substituting FFM for weight. It is also significant to point out that HGS was lower in underweight IPF patients or those with low FFM or low SM. We have also appraised whether muscle strength could be affected by disease severity; quite surprisingly, in line with findings on body composition (see above), we found that HGS did not vary depending on GAP or TORVAN stages, with this finding further confirmed by the weak association found between HGS and lung function.
Dynapenia is defined as the loss of muscle strength associated with aging (or even nutrition-related diseases) not caused by neurological or muscular disorders14; the reduction of muscle strength is not necessarily linked to or caused by a decrease in skeletal muscle mass34. From a clinical point of view, dynapenia (usually recognized as low HGS values) is associated with reduced ability to perform daily living activities, increased morbidity and mortality, and worse quality of life15,16. According to the recent EWGSOP-2 consensus15, dynapenia is the first criterion to consider for the diagnosis of sarcopenia. Considering chronic lung diseases, there are few reports on dynapenia in COPD patients35,36 and none in patients with IPF. Facing this background, one of the main objectives of the present study was to evaluate the prevalence of dynapenia according to various cutoffs proposed by the literature; in all cases, a subject/patient is dynapenic if his/her HGS falls below a defined threshold value. The cutoffs have been derived in different ways: for example, the recent 2019 EWGSOP consensus20 set threshold values based on data published by Dodds et al.32 as mean HGS of young adults minus 2.5 standard deviations (SD), while Lauretani et al.37 referred to the ability of HGS to discriminate elderly individuals with reduced mobility. Consequently, although there is a certain similarity, the selected cutoffs vary from 26 to 33.1 kg for men and from 16 to 21 kg for women (see “Methods” section).
At first glance, mean HGS values of patients with IPF appear to be relatively low; in effect, the prevalence of dynapenia was high, but indeed varied considerably according to the selected criteria both in male(from 20.0 to 51.4%) and female patients (from 25.0 to 68.8%), with a tendency towards higher values in the latter. There was a clear effect of age as dynapenia was significantly more often detected in patients aged 75 years or more; for instance, the prevalence increased from 15.9 to 46.4% with the EWGSOP-2 criteria and from 48.6 to 78.6% with the TESSIER criteria. These findings are not unexpected since a decline of HGS with aging is a well-known phenomenon15,38,while the cutoffs are the same for all subjects. On the other hand, it is worth noting that in most instances, the prevalence of dynapenia did not differ in underweight patients or those with low FFM. Finally, the comparison concerning the severity of disease, dynapenia was more prevalent in the more advanced GAP or TORVAN stages. Overall, as recently stated21, and based on what is known for COPD, the impairment of muscle function in patients with IPF is likely to be multifactorial and due to muscle disuse, hypoxaemia, inflammation, oxidative stress, etc. Dynapenia seems to be a relevant clinical problem especially in older patients.
Limitations of our study include the single-center setting along with the cross-sectional and retrospective design. The study population included patients intercepted at different time points of the disease, and most of them were already on anti-fibrotic therapy. Unfortunately, the sudden occurrence of the COVID-19 pandemic prevented any effort of recruitment and follow-up of new cases. Longitudinal studies will help understand the impact of dynapenia and altered body composition on disease presentation and progression/survival, maybe in combination with the quality of life evaluation. An additional limitation of our study is the lack of data on physical activities. Certainly, future efforts will help address this issue as well. Any inter-relation with anti-fibrotic therapies also merits to be further investigated. With respect to this topic, although we found no associations of either body composition, HGS, and dynapenia with the currently used drugs, nintedanib and pirfenidone, to the best of our knowledge, the present study represents the first attempt in exploring this area. Recently, Jouneau et al. have shown, by retrospectively evaluating the pooled data of the two Inpulsis trials, that the rate of FVC decline was greater in patients with low baseline BMI and those with > 5% weight loss over 52 weeks. Interestingly, nintedanib reduced lung function decline independently of BMI and had a greater effect in patients with > 5% weight loss8. In addition, Suzuki et al. have shown in two retrospective case series that IPF patients under anti-fibrotic therapy had skeletal muscle loss and that sarcopenia was a prognostic factor of reduced survival39.
In conclusion, the present study systematically evaluated HGS in IPF, showing lower values in underweight patients or with low FFM. HGS varied, at least in part, depending on weight, body composition, and especially age. The age-related decline in muscle strength persisted even after correction for body composition. From a clinical point of view, dynapenia (i.e., low muscle strength) was found to be highly prevalent, even more in patients aged > 75 years, although the estimated proportion varied depending on the criteria set used. Overall, the point prevalence estimate of dynapenia tended to be higher in GAP stages II–III than GAP stage I and in TORVAN stages II–III than TORVAN stage I. Finally, no differences emerged in HGS or prevalence of dynapenia by comparing patients treated with nintedanib to those taking pirfenidone.
Based on the evidence gathered, the measurement of HGS appears to be a promising proxy index of muscle function of IPF patients. Further studies are needed to better understand how this variable can best be used in the disease's clinical management and identify that part of patients that requires special attention in terms of applied nutrition and motor rehabilitation. Endpoints of particular interest would be, along with survival, also physical activity, quality of life, disease progression and anti-fibrotic therapies.
Methods
Study population
The study population was composed of 102 (70 males and 32 females) consecutive patients with clinically stable IPF referring to our outpatient clinic from February 1st, 2019, to March 1st, 2020. They included nine treatment naïve IPF patients and 93 patients already on anti-fibrotic therapy with pirfenidone (n = 44) or nintedanib (n = 49). No patients were previously treated or were taking inhaled or systemic corticosteroids. IPF diagnosis was revised in all patients according to the 2018 official diagnostic criteria3. Coexistance of paraseptal/centrolobular emphysema was detected in a small percentage of cases (8%). Exclusion criteria were related to the diagnosis of respiratory diseases other than IPF, acute exacerbation in the four weeks before the study visit, and lung cancer coexistence. Additional exclusion criteria were related to osteo-muscular and neurological disorders or presence of pace-maker/implantable cardioverter defibrillator. Hospitalization in the three months preceding the study visit was reported in no cases. The study was retrospectively conducted in accordance with the amended Declaration of Helsinki and was approved by the local Ethics committee (Federico II University. Registration number: 120/2020). Enrolled patients gave their written informed consent to participate in the study, and all data of interest were anonymously collected into a dedicated database. Spirometry, lung volumes measurement, and determination of the hemoglobin (Hb)-adjusted DLCOsb were performed using a computer-assisted spirometer (Quark PFT 2008 Suite Version Cosmed Ltd, Rome, Italy) according to international standards40,41,42. The 6MWT was performed by trained hospital staff according to guidelines in those patients with a basal peripheral oxygen saturation > 90% in ambient air43. The Borg scale was used to assess the level of dyspnea and muscular fatigue at the beginning and at the end of the test. The GAP (stages I, II and III) was recorded as previously described. Accordingly, the TORVAN (stages I, II, III and IV) score, which is a disease complexity index accounting for comorbidities that impact on IPF prognosis, was evaluated as well44,45.
Anthropometry and body composition
Body weight and stature were measured to the nearest 0.1 kg and 0.5 cm with a mechanical column scale and a stadiometer, respectively (SECA 711 and SECA 220, Hamburg, Germany); BMI was then calculated as body weight (in kg) divided by stature squared (in m2). Body composition was assessed by BIA. Measurements were carried out with a Human Im-Touch analyzer (© DS Medica S.r.l., Milan, Italy) in standardized conditions (i.e., ambient temperature 23–25 °C, fast > 4 h, empty bladder, supine position for at least 10 min before testing). After cleaning the skin surface, the patients were asked to lie down with their legs and arms slightly abducted to avoid any contact between the limbs and the trunk. A standard tetra-polar technique was used: measuring electrodes were placed on wrist and ankle dorsal surface, while injecting electrodes were on the dorsal surface of the hand and the foot, respectively. Impedance (Z) was measured for both the D and ND body side with an electrical current of 800 mA. Concerning BIA-based estimates of body composition, FFM and SM were determined using BIA equations proposed by Rutten et al.31 for patients with COPD and by Jenssen et al.46, respectively. FFMI was calculated as FFM/stature2 and SMI as SM/stature2, while fat mass (FM) was obtained by subtracting FFM from the weight.
Muscle strength
HGS was measured by the same operator following standard procedures using a Dynex dynamometer (MD systems Inc. Ohio USA) to assess the isometric strength of the D and ND arm. Patients were instructed to stand upright with their shoulder adducted and neutrally rotated, elbow fully extended, and forearm and wrist neutrally positioned during the study. A pre-test was done, allowing the patient to become familiar with the instrument. Three measurements were performed for each hand, one minute apart, alternating between the dominant and non-dominant sides47,48. Maximum values were derived for each arm (D-HGS and ND-HGS), and maximum HGS was finally derived as the highest value of six attempts.
Dynapenia
Dynapenia was defined according to six different criteria sets, five of which derived from consensus documents on the diagnosis of sarcopenia: the FRIED49 and LAURETANI37 criteria were proposed by the 2010 EWGSOP consensus50, the ALLEY-1 and ALLEY-2 criteria38 by the FNIH Sarcopenia Project51, and the EWGSOP-2 criteria by the corresponding 2019 consensus15, based on data by Dodds et al.32. In addition, the TESSIER criteria52, which were recently established in a large sample of Canadian population, were also selected. Whatever the criteria used, a subject/patient is dynapenic if his/her HGS falls below a defined threshold value. The cutoff values for various criteria sets were as follows.
Criteria set | MEN | WOMEN |
|---|---|---|
EWGSOP-2 (2019) | < 27.0 kg | < 16.0 kg |
TESSIER (2019) | < 33.1 kg | < 20.4 kg |
ALLEY-1 (2014) | < 26 kg | < 16 kg |
ALLEY-2 (2014) | < 1.0 (calculated as HGS/BMI) | < 0.56 (calculated as HGS /BMI) |
LAURETANI (2003) | < 30 kg | < 20 kg |
FRIED (2001) | BMI ≤ 24 kg/m2: HGS < 29 kg | BMI ≤ 23 kg/m2:HGS < 17 kg |
BMI > 24 to 26 kg/m2:HGS < 30 kg | BMI > 23 to 26 kg/m2: HGS < 17.3 kg | |
BMI > 26 to 28 kg/m2:HGS < 30 kg | BMI > 26 to 29 kg/m2: HGS < 18 kg | |
BMI > 28 kg/m2: HGS < 32 kg | BMI > 29 kg/m2: HGS < 21 kg |
Statistical analysis
Results are expressed as mean ± SD or SE, median value (and interquartile range = IR), and frequency, where appropriate. Statistical significance was pre-determined as p < 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS Inc, Chicago, IL, USA) version 24. ANOVA with the post-hoc Tukey test and the general linear model (GLM) were used to compare groups and assess the effects of factors on a single dependent variable (in the case, even after adjusting for covariates). Partial correlation and multiple regression analysis were utilized to identify predictors of a given dependent variable.
Ethics approval and consent for publication
The Ethics committee approved the study of the Federico II University of Naples, Italy (Registration number: 120/2020; 22/05/2020). All patients signed informed consent.
Data availability
Data are available upon reasonable request.
Abbreviations
- BIA:
-
Bioimpedance analysis
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- D:
-
Dominant side
- D-HGS:
-
Handgrip strength dominant side
- DLCOsb :
-
Diffusion lung capacity for carbon monoxidesingle breath
- FFM:
-
Fat-free mass
- FFMI:
-
Fat-free mass index
- FM:
-
Fat mass
- FVC:
-
Forced vital capacity
- GLM:
-
General linear model
- HGS:
-
Handgrip strength
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IR:
-
Interquartile range
- Maximum HGS:
-
Greatest value for HGS considering both arms
- 6MWT:
-
6 min Walk test
- ND:
-
Non-dominant side
- ND-HGS:
-
Handgrip strength non-dominant side
- SD:
-
Standard deviation
- SE:
-
Standard error
- SEE:
-
Standard error of estimate
- SM:
-
Skeletal muscle
- SMI:
-
Skeletal muscle index
- Z:
-
Impedance
References
Long, R., Stracy, C. & Oliver, M. C. Nutritional care in chronic obstructive pulmonary disease. Br. J. Community Nurs. 23, S18–S26. https://doi.org/10.12968/bjcn.2018.23.Sup7.S18 (2018).
Raad, S., Smith, C. & Allen, K. Nutrition status and chronic obstructive pulmonary disease: Can we move beyond the body mass index?. Nutr. Clin. Pract. 34, 330–339. https://doi.org/10.1002/ncp.10306 (2019).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e44–e68. https://doi.org/10.1164/rccm.201807-1255ST (2018).
Hutchinson, J., Fogarty, A., Hubbard, R. & McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 46, 795–806. https://doi.org/10.1183/09031936.00185114 (2015).
Kreuter, M. et al. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE 11, e0151425. https://doi.org/10.1371/journal.pone.0151425 (2016).
Maher, T. M. & Strek, M. E. Antifibrotic therapy for idiopathic pulmonary fibrosis: Time to treat. Respir. Res. 20, 205. https://doi.org/10.1186/s12931-019-1161-4 (2019).
Faverio, P. et al. Nutrition in patients with idiopathic pulmonary fibrosis: Critical issues analysis and future research directions. Nutrients https://doi.org/10.3390/nu12041131 (2020).
Jouneau, S. et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir. Res. 21, 312. https://doi.org/10.1186/s12931-020-01528-4 (2020).
Kim, J. H., Lee, J. H., Ryu, Y. J. & Chang, J. H. Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc. Respir. Dis. (Seoul) 73, 162–168. https://doi.org/10.4046/trd.2012.73.3.162 (2012).
Alakhras, M., Decker, P. A., Nadrous, H. F., Collazo-Clavell, M. & Ryu, J. H. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 131, 1448–1453. https://doi.org/10.1378/chest.06-2784 (2007).
Jouneau, S. et al. What are the best indicators to assess malnutrition in idiopathic pulmonary fibrosis patients? A cross-sectional study in a referral center. Nutrition 62, 115–121. https://doi.org/10.1016/j.nut.2018.12.008 (2019).
Nishiyama, O. et al. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 22, 480–485. https://doi.org/10.1111/resp.12941 (2017).
Suzuki, Y. et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 8, 14074. https://doi.org/10.1038/s41598-018-32478-z (2018).
Clark, B. C. & Manini, T. M. What is dynapenia?. Nutrition 28, 495–503. https://doi.org/10.1016/j.nut.2011.12.002 (2012).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 601. https://doi.org/10.1093/ageing/afz046 (2019).
Bohannon, R. W., Wang, Y. C., Yen, S. C. & Grogan, K. A. Handgrip strength: A comparison of values obtained from the NHANES and NIH toolbox studies. Am. J. Occup. Ther. https://doi.org/10.5014/ajot.2019.029538 (2019).
Massierer, D., Alsowayan, W., Lima, V. P., Bourbeau, J. & Janaudis-Ferreira, T. Prognostic value of simple measures of physical function and muscle strength in COPD: A systematic review. Respir. Med. 161, 105856. https://doi.org/10.1016/j.rmed.2019.105856 (2020).
Guler, S. A., Hur, S. A., Lear, S. A., Camp, P. G. & Ryerson, C. J. Body composition, muscle function, and physical performance in fibrotic interstitial lung disease: A prospective cohort study. Respir. Res. 20, 56. https://doi.org/10.1186/s12931-019-1019-9 (2019).
Hanada, M. et al. Effect of long-term treatment with corticosteroids on skeletal muscle strength, functional exercise capacity and health status in patients with interstitial lung disease. Respirology 21, 1088–1093. https://doi.org/10.1111/resp.12807 (2016).
Kozu, R., Jenkins, S. & Senjyu, H. Evaluation of activity limitation in patients with idiopathic pulmonary fibrosis grouped according to Medical Research Council dyspnea grade. Arch. Phys. Med. Rehabil. 95, 950–955. https://doi.org/10.1016/j.apmr.2014.01.016 (2014).
Kanjrawi, A. A., Mathers, L., Webster, S., Corte, T. J. & Carey, S. Nutritional status and quality of life in interstitial lung disease: A prospective cohort study. BMC Pulm. Med. 21, 51. https://doi.org/10.1186/s12890-021-01418-5 (2021).
Ebihara, K. et al. Appendicular skeletal muscle mass correlates with patient-reported outcomes and physical performance in patients with idiopathic pulmonary fibrosis. Tohoku J. Exp. Med. 253, 61–68. https://doi.org/10.1620/tjem.253.61 (2021).
Moon, S. W. et al. Thoracic skeletal muscle quantification: Low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 20, 35. https://doi.org/10.1186/s12931-019-1001-6 (2019).
Nakano, A. et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci. Rep. 10, 2312. https://doi.org/10.1038/s41598-020-59100-5 (2020).
Nishiyama, O. et al. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir. Investig. 56, 57–63. https://doi.org/10.1016/j.resinv.2017.09.004 (2018).
Nolan, C. M. et al. Phenotypic characteristics associated with slow gait speed in idiopathic pulmonary fibrosis. Respirology 23, 498–506. https://doi.org/10.1111/resp.13213 (2018).
Sgalla, G. et al. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 19, 32. https://doi.org/10.1186/s12931-018-0730-2 (2018).
Millan-Billi, P., Serra, C., Alonso Leon, A. & Castillo, D. Comorbidities, complications and non-pharmacologic treatment in idiopathic pulmonary fibrosis. Med. Sci. (Basel) https://doi.org/10.3390/medsci6030059 (2018).
Nakatsuka, Y. et al. The clinical significance of body weight loss in idiopathic pulmonary fibrosis patients. Respiration 96, 338–347. https://doi.org/10.1159/000490355 (2018).
Gea, J., Sancho-Munoz, A. & Chalela, R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 10, S1332–S1354. https://doi.org/10.21037/jtd.2018.02.66 (2018).
Rutten, E. P., Spruit, M. A. & Wouters, E. F. Critical view on diagnosing muscle wasting by single-frequency bio-electrical impedance in COPD. Respir. Med. 104, 91–98. https://doi.org/10.1016/j.rmed.2009.07.004 (2010).
Dodds, R. M. et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 9, e113637. https://doi.org/10.1371/journal.pone.0113637 (2014).
de Blasio, F. et al. Raw BIA variables are predictors of muscle strength in patients with chronic obstructive pulmonary disease. Eur. J. Clin. Nutr. 71, 1336–1340. https://doi.org/10.1038/ejcn.2017.147 (2017).
Sampaio, R. A. C., Sewo Sampaio, P. Y., Uchida, M. C. & Arai, H. Management of dynapenia, sarcopenia, and frailty: The role of physical exercise. J. Aging Res. 2020, 8186769. https://doi.org/10.1155/2020/8186769 (2020).
Martinez, C. H. et al. Handgrip strength in chronic obstructive pulmonary disease. Associations with acute exacerbations and body composition. Ann. Am. Thorac. Soc. 14, 1638–1645. https://doi.org/10.1513/AnnalsATS.201610-821OC (2017).
Strandkvist, V. et al. Hand grip strength is associated with fatigue among men with COPD: Epidemiological data from northern Sweden. Physiother. Theory Pract. 36, 408–416. https://doi.org/10.1080/09593985.2018.1486490 (2020).
Lauretani, F. et al. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 1985(95), 1851–1860. https://doi.org/10.1152/japplphysiol.00246.2003 (2003).
Alley, D. E. et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 69, 559–566. https://doi.org/10.1093/gerona/glu011 (2014).
Suzuki, Y. et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 26, 171–179. https://doi.org/10.1111/resp.13943 (2021).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338. https://doi.org/10.1183/09031936.05.00034805 (2005).
Wanger, J. et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 26, 511–522. https://doi.org/10.1183/09031936.05.00035005 (2005).
Macintyre, N. et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 26, 720–735. https://doi.org/10.1183/09031936.05.00034905 (2005).
Laboratories, A. T. S. C. o. P. S. f. C. P. F. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117. https://doi.org/10.1164/ajrccm.166.1.at1102 (2002).
Ley, B. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 156, 684–691. https://doi.org/10.7326/0003-4819-156-10-201205150-00004 (2012).
Torrisi, S. E. et al. The added value of comorbidities in predicting survival in idiopathic pulmonary fibrosis: A multicentre observational study. Eur. Respir. J. https://doi.org/10.1183/13993003.01587-2018 (2019).
Janssen, I., Baumgartner, R. N., Ross, R., Rosenberg, I. H. & Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 159, 413–421. https://doi.org/10.1093/aje/kwh058 (2004).
Gerodimos, V., Karatrantou, K., Psychou, D., Vasilopoulou, T. & Zafeiridis, A. Static and dynamic handgrip strength endurance: Test–retest reproducibility. J. Hand Surg. Am. 42, e175–e184. https://doi.org/10.1016/j.jhsa.2016.12.014 (2017).
Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 40, 423–429. https://doi.org/10.1093/ageing/afr051 (2011).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156. https://doi.org/10.1093/gerona/56.3.m146 (2001).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on sarcopenia in older people. Age Ageing 39, 412–423. https://doi.org/10.1093/ageing/afq034 (2010).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558. https://doi.org/10.1093/gerona/glu010 (2014).
Tessier, A. J., Wing, S. S., Rahme, E., Morais, J. A. & Chevalier, S. Physical function-derived cut-points for the diagnosis of sarcopenia and dynapenia from the Canadian longitudinal study on aging. J. Cachexia Sarcopenia Muscle 10, 985–999. https://doi.org/10.1002/jcsm.12462 (2019).
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.B. and L.S.: conceptualization, investigation, data curation and formal analysis, writing-original draft, review and editing. P.A. and L.C.: investigation, methodology, data curation and formal analysis; L.G., A.A.S. and A.D.G.: investigation, methodology and data curation; G.R.: methodology, imaging data curation and review; A.S.Z.: conceptualization and review. All authors have read and approved the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bocchino, M., Alicante, P., Capitelli, L. et al. Dynapenia is highly prevalent in older patients with advanced idiopathic pulmonary fibrosis. Sci Rep 11, 17884 (2021). https://doi.org/10.1038/s41598-021-97424-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97424-y
This article is cited by
-
Phase angle in assessment and monitoring treatment of individuals with respiratory disease
Reviews in Endocrine and Metabolic Disorders (2023)
-
Sarcopenia in idiopathic pulmonary fibrosis: a prospective study exploring prevalence, associated factors and diagnostic approach
Respiratory Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.