Abstract
Study design:
Case report
Objective:
To describe the clinical and imaging findings of a patient with painless aortic dissection.
Setting:
University Neurology Department, Thessaloniki, Greece
Patient, Methods, Results:
A 46-year-old man was transferred to our Department for emergent evaluation of paraplegia, from the local hospital of the nearby town, where he was admitted complaining from sudden, painless, bilateral leg weakness, 24 h earlier. He presented complete flaccid paraplegia with urinary retention, loss of pain and temperature sensation below the TH7 level and well-preserved vibration and position sense bilaterally. He had no pain and general physical examination was unremarkable. Chest X-rays first raised the suspicion of an aortic lesion. Thoracic MRI revealed cord dilation, with no enhancement on T1-weighted images (wi) and increased signal on T2-wi at the TH9–TH12 levels, suggesting cord ischemia. At the same MR sequences, the double lumen of the descending aorta indicated dissection in both sagittal and axial images. Later the same day, the patient died, and autopsy verified dissection of the descending aorta up to the aortic valve.
Conclusion:
The rapid evolution of our case further points out that radiologists, neurologists, as well as internal specialists should be vigilant for this emergency, which despite rich imaging could have a fatal outcome.
Similar content being viewed by others
Case report
A 46-year-old man was admitted to the local hospital of a nearby town, complaining for sudden, painless, bilateral leg weakness while defecating, on October 29. There was no family history of any disease and his past medical history disclosed ankylosing spondyloarthritis (positive HLA B27) diagnosed 20 years ago and hypertension treated for the last 2 years. An urgent brain and lumbar spine CT scan was unremarkable and he was transferred to our Department for further evaluation the next day.
Neurologically the patient was alert, fully oriented with normal cranial nerves, upper limbs strength and reflexes. He presented complete flaccid paraplegia with urinary retention, absent plantar and deep tendon reflexes, a spinothalamic thoracic (TH) sensory level at TH7 with loss of pain and temperature sensation below this level, extending over the trunk and both lower limbs. However, he had well-preserved vibration and position sense bilaterally. He was afebrile and complained of no pain, the electrocardiogram was normal, his arterial blood pressure was 140/80 mm Hg, heart rate 82 min−1 with sinus rhythm and no murmurs or other findings were found on general physical examination.
Chest X-rays showed mediastinal enlargement first raising the suspicion of an aortic lesion. Complete blood count and routine serum biochemistry provided normal results, except for increased enzymes LDH 714 U l−1 (normal range 240–480), SGOT 95 U l−1 (range 0–38) and CPK 2866 U l−1 (range 0–190). Emergent lumbar puncture in the lateral decubitus position provided normal cerebrospinal fluid opening pressure (160 mm H2O) and cytochemistry (clear, no cells protein 45 mg per 100 ml, glucose 60 mg per 100 ml and lactate 1.6 meq l−1).
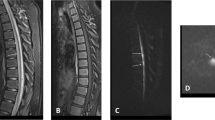
Thoracic spinal cord magnetic resonance imaging revealed cord dilation at the TH9–TH12 level on sagittal T1-weighted images (wi) with no enhancement (Figure 1a) and increased signal on T2 wi at the same levels (Figure 1b). At the same MR sequences, ventrally to the vertebral bodies, the double lumen of the dilated descending aorta indicated dissection (Figures 1a and b). Axial T1 with contrast and T2 wi duplicated both the spinal cord and aortic findings (Figures 1c and d).
Magnetic resonance imaging of the thoracic spinal cord revealed cord dilation at the TH9–TH12 level (arrows) on sagittal T1 wi, with no gadolinium enhancement (a) and increased signal on T2 wi (b) at the same levels. Ventrally to the vertebral bodies, the double lumen of the descending aorta (arrowhead) indicated dissection (a and b). Axial images at TH10 level duplicated both the spinal cord and aortic findings on T1 with contrast (c) and T2 wi (d). wi, weighted images.
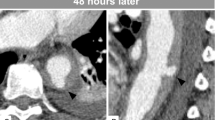
Later the same day the patient died, 1 h before undergoing vascular reconstruction. Autopsy unveiled hemopericardium as the cause of death, secondary to dissection of the descending aorta up to the aortic valve (Figure 2). Extensive serological and virological tests (including protein electrophoresis, Lyme disease, syphilis, herpes viruses and HIV) performed on blood and cerebrospinal fluid samples obtained on admission were completed several days after his death and provided normal results.
Comment
Aortic dissection (AD) is a life-threatening vascular emergency with a wide and extremely variable range of presenting clinical features.1 A history of hypertension is considered the most common predisposing factor of AD and is present in 62–78% of patients,2, 3 who typically present with abrupt thoracic pain.3 The correct diagnosis is often challenging, it can be difficult and delayed especially in pain-free dissections with predominant neurological symptoms,1, 2, 4 as happened in our case. Despite major advances in diagnostic imaging and progress in surgical management, the mortality rate is high,2, 3 the diagnosis is missed on initial evaluation in up to 38% of patients2 and is first established at autopsy in up to 28%.2, 3
Clinical manifestations of spinal cord involvement in patients with AD comprise anterior spinal cord syndrome as happened in our case, as well as transverse myelitis with complete spinal cord infarction, Brown–Sequard syndrome, progressive myelopathy or transient spinal cord ischemia.1, 2, 5
The clinical profile of our patient consisted of an abrupt, painless and rapid development of anterior spinal cord syndrome.2, 5 Immediate magnetic resonance imaging findings on T1 and T2 wi concomitantly indicated dissection of the descending aorta1, 2 and early ischemic changes of the lower TH spinal cord.6 This temporal evolution of clinical and imaging findings strongly supported a direct correlation between AD and spinal cord ischemia in accordance with recent literature data.1, 2, 4
Other possible etiologies of acute myelopathy (compression, infection, inflammation) were excluded by the emergently performed laboratory examinations and finally autopsy-verified extensive AD.
Although spinal cord ischemia on the basis of AD is considered rare,1, 4 the clinical and imaging findings of our case further point out that once the condition is suspected, time is a critical factor and radiologists, neurologists as well as internal specialists should be vigilant for this emergency, especially in atypical presentations.1
References
Gaul C, Dietrich W, Erbguth FJ . Neurological symptoms in aortic dissection: a challenge for neurologists. Cerebrovasc Dis 2008; 26: 1–8.
Khan IA, Nair CK . Clinical, diagnostic and management perspectives of aortic dissection. Chest 2002; 122: 311–328.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283: 897–903.
Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ . Neurological symptoms in type A aortic dissections. Stroke 2007; 38: 292–297.
Beggs AD, Al-Rawi H, Parfitt A . Chest pain fleeting neurological signs. Lancet 2005; 365: 1514.
Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE . Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology 2002; 44: 851–857.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karacostas, D., Anthomelides, G., Ioannides, P. et al. Acute paraplegia in painless aortic dissection. Rich imaging with poor outcome. Spinal Cord 48, 87–89 (2010). https://doi.org/10.1038/sc.2009.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.70
Keywords
This article is cited by
-
Dissection aortique révélée par une paraplégie brutale isolée
Annales françaises de médecine d'urgence (2013)