Abstract
Study design:
Experimental animal study.
Objective:
To evaluate motor and sensitive axonal regrowth after multiple intercosto-lumbar neurotizations in a sheep model.
Setting:
France.
Methods:
Fifteen sheep were separated into three groups. Five sheep had multiple intercosto-lumbar neurotizations and a spinal cord lesion, five sheep were neurotized without any spinal cord lesion and five sheep had a spinal cord lesion without any neurotizations. Six months after the initial surgery, histological study of the neurotized roots was performed.
Results:
The length of the three rerouted intercostal nerves was sufficient in the 10 sheep to perform an intercosto-lumbar neurotization in good conditions. Eight sheep out of the 15 had postoperative complications responsible for the animal's death in five cases. Histological cross-sections of all the neurotized L2, L3 and L4 roots showed numerous myelinated regenerated axons. Dorsal root ganglions of neurotized roots showed both large and small neurons with normal nucleus and cytoplasm. The fluorescent retrograde labeling of 18 roots revealed labeled motor neurons in five cases.
Conclusions:
This study demonstrates the technical feasibility of intercosto-lumbar neurotizations in a big mammalian model. Intercostal nerve harvesting and rerouting was successfully performed in all the cases. Our histological results proved, in all the animals studied, the ability of motor and sensitive neurons to regenerate through the neurotization area. In the context of the future clinical application of strategies aimed at promoting axonal regeneration after severe spinal cord injury, the present data suggest that multiple intercosto-lumbar neurotization could be helpful to promote lower limb muscular strength recovery after spinal cord injuries.
Similar content being viewed by others
Introduction
The loss of neurological function after complete spinal cord injury remains a devastating condition. Despite initial medical and surgical care, including systemic delivery of high-dose methylprednisolone and early reduction, decompression, and stabilization,1, 2, 3 little hope for substantial recovery of neurological function in either the acute or the chronic setting is offered to patients. Since the recognition 20 years ago of the inherent capacity of central nervous system axons to regenerate, much progress has been made in elucidating the mechanisms that facilitate or inhibit axon regeneration.4, 5, 6, 7 Despite these advances, functional recovery after spinal cord injury in experimental models has been insufficient to bring any regenerative therapy to clinical trials. Therefore, in addition to cellular neurobiology and tissue engineering strategies, bypass techniques in the form of intercostal neurotizations have been proposed by some authors.8, 9, 10, 11, 12, 13, 14 Neurotization of lumbar roots with lower intercostal nerves is a potential way to treat neurological deficits after spinal cord injury.8, 10, 15, 16, 17 The sheep spine is a useful model for experiments evaluating bony or neurological structures of the thoracic and lumbar spine. We have devised an experiment to examine the feasibility of neurotization of three lumbar roots using lower intercostal nerves after hemisection of the sheep spinal cord. The anatomical feasibility of such neurotizations in humans10, 16 and the feasibility of electrophysiologically monitoring lumbar roots in a sheep model18 has already been reported. However, axonal regrowth after intercostal to lumbar neurotization needs to be assessed in a large mammalian model. The aim of this study is to evaluate motor and sensitive axonal regrowth after multiple intercosto-lumbar neurotizations in a sheep model.
Materials and methods
Technique
Experiments were conducted on 15 Prealpes du Sud male adult sheep aged from 12 to 14 months. They were free from ecto- and endoparasites, Mycoplasma pulmonis and Pasteurella (P. multocida and P. pneumotropica). Animals were supplied by van in individual cages and acclimatized for 1 week in the department of animal surgery at the Ecole de chirurgie de l’Assistance Publique des Hôpitaux de Paris. Animals were housed during the experiment in individual galvanized wire cages (80 × 120 × 160 cm). The room was equipped with locks and fluorescent lighting (300 Lux at 1 m above floor) and the temperature and humidity regulated (24±2 °C; 55±10%). Animals had unrestricted access to food (forage and grass) and untreated drinking water.
All animals received humane care in accordance with the scientific Committee on Animal Research at the Ecole de Chirurgie de l’Assistance Publique des Hôpitaux de Paris, which approved the study. These animals were not used in any other experimental protocol.
Positioning and anesthesia
The animals were placed in a sternal position. General anesthesia was induced with thiopental (bolus of 1.0 g per sheep) and maintained with halothane (1–2%) in a mixture of nitrous oxide (0.5–1 l min−1) and oxygen (1.5 l min−1). Each animal underwent positive pressure ventilation. The rate and end tidal pressure were adjusted to maintain an end tidal pCO2 of 40±2 mm Hg.
Surgical procedure
The 15 sheep were separated into three groups (Figure 1). In group A, five sheep had a left T11-L2, T12-L3 and T13-L4 neurotization. In group B, five sheep had a left T11-L2, T12-L3 and T13-L4 neurotization and a L1 level left hemisection of the spinal cord performed during the same time. In group C (Control group), five sheep had a left L2, L3 and L4 section and a L1 level left hemisection of the spinal cord.
Surgical procedure (nerve harvesting and intercosto-lumbar neurotizations)
A 25-cm midline incision was made posteriorly over the spinous processes of T10-L5 and continued cranially and laterally to expose the left rib (Figure 2). The ramus cutaneus lateralis of the eleventh, twelfth and thirteenth intercostal nerves were isolated and sectioned just after their exits from the musculus intercostalis externus. The dissection of deep medial branches of the intercostal nerves were continued as distally as possible (Figure 3). The intermediate part of each nerve between its medial and lateral approach was dissected. After release of the intermediate part of the nerve, it was taken in a postero-medial direction. The three sectioned intercostal nerves were rerouted through a lateral para-spinal route to reach the lateral aspect of the L2, L3 and L4 vertebral isthmus.
After section of the L2, L3 and L4 roots, a neurotization of L2 by T11, L3 by T12 and L4 by T13 was performed (Figure 4). The end-to-end nerve anastomosis was performed using 0.3 ml of fibrin glue (Tissuecol duo, Baxter, Unterschleissheim, Germany).
In groups B and C, after releasing the vertebral posterior arch and opening the dura mater at the L1 level, a left hemisection of the spinal cord was performed. The dural incision was closed by a continuous resorbable suture.
At the end of each surgical procedure, the wound was closed by conventional technique without a suction drain. During the first 10 postoperative days, animals were housed in individual cages with unrestricted access to food (forage and grass) and untreated drinking water. The sheep had a clinical follow-up every month during 6 months.
Histological study
Six months after the initial surgery, a retrograde labeling of the neurotized roots was performed. Section of the root was done 2 cm distal to the neurotization site. L2 roots were labeled with 10 μl of blue neuronal tracer (Cascade Blue, Invitrogen, 95613 Cergy Pontoise Cedex, France), L3 roots were labeled with 10 μl of red neuronal tracer (Fluoro-Ruby, Invitrogen) and L4 roots were labeled with 10 μl of yellow neuronal tracer (Lucifer Yellow, Invitrogen). Ten days after the labeling procedure, the animals were killed by intravenous injection of sodium pentobarbital. The sectioned spinal cord was fixed in 4% paraformaldehyde and cryoprotected with 30% sucrose at 41 °C. Serial sections (40 μm) of dorsal root ganglia (DRG) and spinal cord from T11 to T13 were cut on a freezing microtome and mounted on gelatin-coated glass slides. These slides were examined with a fluorescence microscope (Carl Zeiss, Le Pecq, France) equipped with appropriate triple-filter sets. Photographs were taken using a digital camera. Other serial sections (1 μm) of DRG and spinal cord were fixed with paraffin, stained with thionin and coverslipped before examination using a conventional light microscope.
Results
Surgical procedure
Thoracic and lumbar roots were easily identified in all cases. The lumbar roots, surrounded by anastomotic vessels were easily located at the lateral aspect of vertebral isthmus. Before section of the lumbar roots at their exit from the vertebral foramen, preventive hemostasis was mandatory to avoid perioperative bleeding from vertebral foramen vessels.
The length of the three rerouted intercostal nerves was sufficient in the 10 sheep to perform an intercosto-lumbar neurotization in good conditions.
Clinical results and complications
In groups A and B, a flaccid paralysis of the left abdominal wall was noted 3 months after the surgery (Figure 5a). The asymmetry of the abdominal wall improved in all the cases at the 6-month follow-up (Figure 5b). In groups B and C, the left lower limb flaccid paralysis did not improve at follow-up. In group C, the asymmetry of the abdominal wall remained unchanged.
Eight sheep out of the 15 had postoperative complications. Three had early respiratory complications responsible for the animal's death. In two cases, a pneumothorax was diagnosed and pleural drainage performed. Despite these procedures, the animals died from respiratory compromise 5–9 days after surgery. One sheep in group A experienced an early wound dehiscence, which was successfully treated by wound debridement and closure. Two sheep in group C had large pressure sores because of flaccid paralysis of the left lower limb. In these two cases the decision was to kill the animals by intravenous injection of sodium pentobarbital. Two sheep suffered from a late undiagnosed complication resulting in death 2.5 and 5 months after surgery.
The details of the clinical follow-up and complications are summarized in Table 1.
Histological results
Light microscopic analysis
Under the light microscope, the semithin cross-sections of all the neurotized L2, L3 and L4 roots showed numerous myelinated axons, mostly clustered (Figure 6a). A rich neovascularization was noted. In control animals (group C), complete degeneration without regeneration was noted on the semithin cross-sections of the sectioned and untreated roots. The semithin cross-sections of neurotized T11, T12 and T13 DRG showed both large and small neurons with normal nucleus and cytoplasm (Figure 6b). The semithin cross-sections at the T11, T12 and T13 levels showed a normal population of motor neuron without any sign of spinal cord degeneration. In control animals (group C), a degeneration of DRG composed of a small number of nonspecific cells was noted on the semithin cross-sections.
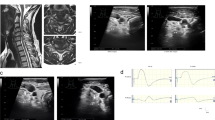
(a) Semithin cross-sections of a T11L2 neurotized root showing numerous myelinated axons, mostly clustered (magnification × 40). (b) Semithin cross-sections of a T12-L3 neurotized dorsal root ganglion showing both large and small neurons with normal nucleus (N) and cytoplasm (magnification × 64). A full colour version of this figure is available at the Spinal Cord journal online.
Fluorescence microscopic analysis
The use of fluorescent retrograde markers in six sheep (18 roots) was quite disappointing. Our main intention was to find out whether in neurotized lumbar roots, motor and DRG neurons were labeled, indicating the presence of a connectivity between peripheral nerve and the spinal cord. We were able to verify this projection in two out of the three different tracers used in this study. Axonal fibers in neurotized roots have picked up the tracer and, by retrograde transport, a small cohort of motor neuron bodies were backfilled by the tracer at the anterior horn of the spinal cord. The labeling of 18 roots revealed labeled motor neurons in five cases, only with yellow and blue tracers (Figures 7a and b). We did not find retrograde labeling of cellular bodies with the red (Fluoro-ruby) tracer. Despite numerous additional frozen slices of T11, T12 and T13 DRG, fluorescent labeling of small and large DRG neurons was not found.
Discussion
Serious injuries of the spinal cord often result in permanent deficits below the lesion level. To regain function lost after such damage, the upstream axons must reinnervate the denervated territory. Therefore, the regrowth of the axons rostral to the lesion must be initiated, and the growing fibers must be provided a means by which they can traverse or surround the lesion site. Multiple lumbar root neurotizations with lower intercostal nerves have been proposed by some authors as being a new surgical procedure in order to treat neurological deficits after spinal cord lesions.8, 15, 17, 19, 20 Using this technique, the use of the spinal cord and intercostal nerves above the spinal cord lesion avoids axonal regrowth having to go through the injured central nervous system. As a consequence, only the lesions of the cauda equina and conus medullaris can be treated by such lower intercostal nerve neurotizations. Although brachial plexus neurotization may provide some clinically meaningful benefit (for example, shoulder animation, elbow flexion), the size of the intercostal nerves and the distance of the muscle endplate from the proximal cauda equina could lead to unsatisfactory results with no ‘clinically meaningful’ somatic leg function recovery.
Lang et al.17 have, however, recently reported their use of lower intercostal nerves for neurotization of the lower limb in humans. According to the recent preliminary clinical applications reported by Livshits et al.15 and Sievert et al.,20 a potentially more viable strategy may be through neurotization of the sacral plexus to improve bladder and sexual dysfunction after spinal cord lesions.
In light of these recent studies, we performed this animal study to demonstrate axonal regeneration and functional improvement using this surgical procedure.
This study demonstrates the technical feasibility of intercosto-lumbar neurotizations in a big mammalian model. Intercostal nerve harvesting and rerouting was successfully performed in all the cases. Our histological results proved, in all the animals studied, the ability of intercostal nerves to regenerate through the neurotization area down to the muscular effectors. The clinical consequence of the muscle strength recovery was an improvement of the abdominal wall asymmetry in all the cases. Because neurotizations were performed immediately after the denervation, our results need to be considered in the context of poor axonal regeneration in chronically denervated muscles. The mechanisms by which protection may mediate the positive or negative effects on the regenerative capacity of axotomized neurons also need to be taken into account.
In preliminary studies, electrophysiological investigations were conducted to assess nerve tract continuity from the central nervous system (the spinal cord) to the muscular or sensory effectors.18 Unfortunately, in this study, the use of fluorescents tracers was disappointing. The low number of neurons marked with the traced could be as a consequence of a mistake in the delay between labeling and spinal cord harvesting. Previous experiments with such fluorescent tracers were performed on small animals (mice or rats) with a 2–3 days delay between the labeling and the harvesting procedures. Despite the 10 days delay used in our study, this time could be shorter to obtain a retrograde labeling of cytoplasm in both sensory or motor neurons. We postulate that the quantity (10 μl) of neuronal tracer could also be insufficient to obtain a distinguishable cellular fluorescence.
As a consequence, the sensory effector to neuron continuity was assessed by light microscopy of the DRG. Because of the normal architecture of DRG (which is composed of both large and small neurons with normal nucleus and cytoplasm) we concluded that the axonal regrowth of the sensory tract was effective in the A and B groups.
The goal of this work was to report the technical feasibility and results of multiple neurotizations of lumbar roots with lower intercostal nerves in a sheep model. We performed such neurotizations without technical difficulties due to the size of the model combined with the harvesting technique. The harvesting technique was reproducible as in previous anatomical studies performed on human cadavers.10, 16
However, we noted a high rate of postoperative complications, especially due to pleural damage and respiratory compromise. We think that pleural damage could occur during intercostal nerve harvesting because of the nerve course, close to the parietal pleura. Unfortunately, the use of a pleural suction drain seemed not to be easy in routine practice in this model, as the sheep have a predilection for eating the drain. In these conditions, we concluded that sheep model was not the easiest to use assessing multiple intercosto-lumbar neurotization procedures.
In the context of the future clinical application of strategies aimed at promoting axonal regeneration after severe spinal cord injury, the present data suggest that multiple intercosto-lumbar neurotization could be helpful to promote lower limb muscular strength recovery after spinal cord injuries. Specific axonal tracing holds promise of being a useful technique to examine sensory and motor pathway recovery after neurotization in our sheep model. However, quantities of tracer as well as the delay between labeling and harvesting need to be reconsidered. Further studies using this technique are currently being conducted at our department.
References
Fehlings MG, Sekhon LH, Tator C . The role and timing of decompression in acute spinal cord injury: what do we know? What should we do? Spine 2001; 26: S101–S110.
Fehlings MG, Tator CH . An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg 1999; 91: 1–11.
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990; 322: 1405–1411.
Richardson PM, McGuinness UM, Aguayo AJ . Axons from CNS neurons regenerate into PNS grafts. Nature 1980; 284: 264–265.
McKerracher L . Spinal cord repair: strategies to promote axon regeneration. Neurobiol Dis 2001; 8: 11–18.
Fawcett JW . Spinal cord repair: from experimental models to human application. Spinal Cord 1998; 36: 811–817.
David S, Aguayo AJ . Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science 1981; 214: 931–933.
Zhao S, Beuerman RW, Kline DG . Neurotization of motor nerves innervating the lower extremity by utilizing the lower intercostal nerves. J Reconstr Microsurg 1997; 13: 39–45.
Zhang S, Johnston L, Zhang Z, Ma Y, Hu Y, Wang J et al. Restoration of stepping-forward and ambulatory function in patients with paraplegia: rerouting of vascularized intercostal nerves to lumbar nerve roots using selected interfascicular anastomosis. Surg Technol Int 2003; 11: 244–248.
Vialle R, Court C, Harding I, Lepeintre JF, Khouri N, Tadié M . Multiple lumbar plexus neurotizations of the ninth, tenth, and eleventh intercostal nerves. Clin Anat 2005; 19: 51–58.
Tok S, Schmid UD, Ferbert A, Davenport T . Intercostolumbar spinal nerve anastomosis. An experimental study in dogs. Spine 1991; 16: 463–466.
Okinaga S, Nagano A . Can vascularization improve the surgical outcome of the intercostal nerve transfer for traumatic brachial plexus palsy? A clinical comparison of vascularized and non-vascularized methods. Microsurgery 1999; 19: 176–180.
Cheng H, Shoung HM, Wu ZA, Chen KC, Lee LS . Functional connectivity of the transected brachial plexus after intercostal neurotization in monkeys. J Comp Neurol 1997; 380: 155–163.
Asfazadourian H, Tramond B, Dauge MC, Oberlin C . Morphometric study of the upper intercostal nerves: practical application for neurotizations in traumatic brachial plexus palsies. Chir Main 1999; 18: 243–253.
Livshits A, Catz A, Folman Y, Witz M, Livshits V, Baskov A et al. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord 2004; 42: 211–217.
Court C, Vialle R, Lepeintre JF, Tadié M . The thoracoabdominal intercostal nerves: an anatomical study for their use in neurotization. Surg Radiol Anat 2005; 27: 8–14.
Lang EM, Borges J, Carlstedt T . Surgical treatment of lumbosacral plexus injuries. J Neurosurg Spine 2004; 1: 64–71.
Vialle R, Loureiro M, Ilharreborde B, Liu S, Lozeron P, Tadié M . The feasibility of detecting motor and sensory potentials in a sheep model. Lab Animals 2006; 40: 469–473.
Hauge EN . The anatomical basis of a new method for re-innervation of the gluteal region in paraplegics. Acta Physiol Scand Suppl 1991; 603: 19–21.
Sievert KD, Xiao CG, Hennenlotter J, Seibold J, Merseburger AS, Kaminskie J et al. Voluntary micturition after intradural nerve anastomosis. Urologe A 2005; 44: 756–761.
Hems TE, Clutton RE, Glasby MA . Repair of avulsed cervical nerve roots. An experimental study in sheep. J Bone Joint Surg Br 1994; 76: 818–823.
Hems TE, Glasby MA . Repair of cervical nerve roots proximal to the root ganglia. An experimental study in sheep. J Bone Joint Surg Br 1992; 74: 918–922.
Falempin M, Rousseau JP . Reinnervation of skeletal muscles by vagal sensory fibres in the sheep, cat and rabbit. J Physiol 1983; 335: 467–479.
Acknowledgements
This study profited from the financial support of the ‘Société Française de Chirurgie Orthopédique et Traumatologique’ and of the ‘Fondation pour la Recherche Médicale’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vialle, R., Lacroix, C., Harding, I. et al. Motor and sensitive axonal regrowth after multiple intercosto-lumbar neurotizations in a sheep model. Spinal Cord 48, 367–374 (2010). https://doi.org/10.1038/sc.2009.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.144