Abstract
Study design:
A cross-sectional design.
Objectives:
To examine the length–tension relationship of dorsiflexion (DF) and plantarflexion (PF) muscle groups in seven individuals with chronic spinal cord injury (SCI; C2–T7; age 43±10.1 years) and compare it with a group of age and sex-matched able-bodied (AB) controls.
Setting:
McMaster University, Hamilton, ON, Canada.
Methods:
Isometric single twitch properties, 0.5-s tetanic contractions (SCI) and maximal voluntary contractions (AB) were measured at nine joint angles from 20° DF to 20° PF.
Results:
In the DF muscles, peak-evoked twitch (PT) torque occurred at 20° PF for SCI (3.4±1.1 N m) and AB (3.8±1.4 N m) with no difference in peak torque between groups, whereas peak summated force occurred at 10° PF in AB and 20° PF in SCI (P<0.01). In the PF muscles, PT torque occurred at 15° DF in AB (18.6±2.6 N m) and at 5° DF (6.8±3.3 N m; P<0.01) in SCI, and peak-summated force occurred at 15° DF in AB. The SCI group did not show any change in PF peak-summated force with varying joint angles. Rates of contraction and relaxation were not different between the two groups.
Conclusions:
The results suggest a significant change in the length–tension relationship of the PF muscles after SCI, but no change in the DF muscle group. Rehabilitation programs should focus on maintaining PF muscle length in order to optimize muscle strength and function after SCI.
Similar content being viewed by others
Introduction
After a spinal cord injury (SCI), it has been established that there are significant changes in skeletal muscle properties below the level of the lesion. These include fibre-type transformation,1, 2 atrophy,1 faster contractile properties2, 3, 4 and a decreased range of motion.5 These changes, which can be attributed to both paralysis and a loss of functional innervation, greatly diminish the force-generating capacity of the muscle. Another factor known to affect force generation is muscle length. This phenomenon, known as the length–tension relationship, is attributed to the degree of overlap between the actin and myosin filaments within the myofibrils.6 A major limitation in the majority of studies that have examined various muscle property changes after SCI is the assumption that there is no change in the length–tension relationship. A change in this relationship would not only affect muscle characteristics but would also imply a change in the optimal angle for force generation.
There is good reason to hypothesize that the length–tension relationship might change after SCI. Paralysis is not only associated with muscle atrophy, it is also accompanied by increased connective tissue within the muscle belly7 and possibly a loss of sarcomeres due to prolonged immobilization.8, 9 This increase in connective tissue contributes to a loss of elasticity, an increase in passive tension and a decrease in range of motion.7, 8, 9, 10
To date, only two studies have attempted to address changes in the length–tension relationship after SCI. Gerrits et al.11 found no change in the torque–angle relationship of human knee extensors compared with controls, although the SCI participants produced significantly less torque (relative to their own maximum) at extreme angles. McDonald et al.5 examined length–tension properties of the ankle muscles after SCI and found a significant shift towards plantarflexion in the torque–angle curve of the gastrocnemius/soleus. The tibialis anterior showed no significant shift when comparing the two groups. This study used only single twitch data for an assessment of the torque–angle relationship, and did not report findings related to muscle contractile speed. In addition, an ideal angle for torque development was not identified in either muscle groups.
The purpose of this study was to determine the optimal angle for force production of dorsiflexor (DF) and plantarflexor (PF) muscle groups after SCI. It was predicted that the optimal angles for peak twitch and maximal summated force production would occur at shorter muscle lengths for the SCI group versus able-bodied (AB) controls. It was also predicted that at all muscle lengths, participants with SCI would produce significantly less torque compared with controls. Finally, given the purported shift towards faster fibre types after SCI, it was predicted that the SCI group would display faster contractile speeds for both the DF and PF muscle groups.
Materials and methods
Subjects
Seven male subjects with SCI (43±10.1 years) and seven age and sex-matched AB control subjects (42±10.8 years) were used as participants in this study. All participants were recreationally active, and none had participated in any form of muscle stimulation training. SCI subject characteristics are summarized in Table 1. Inclusion criteria included: >1 year post-injury, upper motor lesion level above T8, American Spinal Injury Association (ASIA) classification A–C and wheelchair dependent. Testing procedures were approved by the McMaster University Research Ethics Board and an informed consent was obtained from each participant.
Apparatus
All measurements were conducted on the right ankle DF and PF muscles. Subjects either remained in their wheelchair (SCI) or sat on a height-adjustable chair (AB) with the right knee flexed at 90°. The lower leg and foot was secured in an isometric boot apparatus. For the DF muscle group, rubber stimulating electrodes (3 cm diameter) were applied onto clean abraded skin over the common peroneal nerve just distal to the proximal head of the fibula and on the anterior aspect of the leg, slightly distal to the patella. The PF muscle group was stimulated in the popliteal fossa overlying the tibial nerve with two lead plate stimulating electrodes imbedded in a plastic housing. All electrodes were coated in conducting gel before placement on the skin and secured with tape or a velcro strap. Muscle electrical activity was collected using two surface electromyogram electrodes (Kendall Medi-Trace 133, Kendall Healthcare, Mansfield, MA, USA) placed 5 cm apart on the muscle belly of the tibialis anterior and soleus muscles. A ground electrode was secured on the lateral aspect of the lower leg, between the stimulating and recording electrodes.
A high-voltage constant current stimulator (Digitimer, Model 3072, Digitimer Ltd, Welwyn Garden City, Hertfordshire, England) was used to deliver single 50-μs rectangular pulses and 0.5-s tetanic trains (80 Hz) to the peroneal and tibial nerve for assessment of peak-evoked twitch (PT) and peak tetanic torque (TT), respectively. The stimulation frequency (80 Hz) was selected based on the force–frequency relationship for the tibialis anterior in people with SCI previously determined in our lab (unpublished). The optimal stimulation intensity was determined at 15° PF for the DF muscle group and at 15° DF for the PF muscle group. For each participant, stimulation current was gradually increased until there were no further increases in PT. This optimal stimulation intensity was then used for assessment of the PT and TT at different joint angles. Data were acquired using custom-designed software (Labview, National Instruments; Austin, TX, USA).
Procedure
After being secured in the boot apparatus and determining optimal stimulation intensity, muscle contractile properties in the PF and DF muscles were measured at nine joint angles, ranging from 20° DF to 20° PF in 5° increments. The muscle contractile properties measured were PT, maximum compound action potential amplitude (M-wave), time to PT (TPT), maximum rate of torque development (RTD), ½ relaxation time and maximum rate for torque relaxation (RTR). The DF muscle group was assessed first followed by the PF group, and the joint angle order was randomized. For the determination of maximum summated force-generating capacity in the SCI group, a 0.5-s tetanic train at 80 Hz was evoked at each joint angle after the assessment of PT to provide peak TT. Alternatively, in the AB group, subjects were instructed to produce a 5 s maximum voluntary contraction (MVC) at each angle after all the single twitch data were collected to avoid any potentiation effects.
Statistical treatment
Muscle contractile property data were analysed using a two-way mixed analysis of variance (ANOVA) with one between subjects factor (SCI versus AB) and one within subjects factor (joint angle). Significance was set at P<0.05, and Tukey A post-hoc tests were used to examine significant differences between means. Throughout the text, values are reported as mean±s.d.
Results
Effect of joint angle on PT
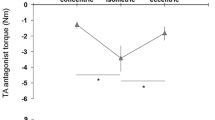
Table 2 presents the contractile properties for both muscles tested. In the DF muscle group, there were no group differences in PT (P=0.49), nor was there a significant group × angle interaction (P=0.71). Maximal PT occurred at 20° PF in both the SCI and AB groups with values of 3.4±1.1 N m and 3.8±1.4 N m, respectively (Figure 1). Despite the similar PT, the evoked twitches in the SCI group were faster; time to PT at the optimal joint angle was 75.4±13.8 ms in SCI compared with 79.9±7.6 ms in AB (P=0.037; Table 2). Although there was a trend towards a faster RTD in SCI at some joint angles (for example, 114.8±36.6 ms in SCI and 87.0±36.3 ms in AB at 15° PF), the difference did not reach significance. Similarly, rates of torque relaxation were not different between the two groups.
In the PF muscles, there were differences between groups in both the magnitude of PT and the optimal angle for evoked force generation (P<0.01; Figure 1). The optimal angle for PT occurred at 5° DF in the SCI group (6.8±3.3 N m) and at 15° DF in AB group (18.6±2.5 N m). As seen in Table 2, time to PT was faster in SCI (62.4±18.8 ms) compared with AB (116.2±14.8 ms; P<0.00) due to the significantly smaller PT, whereas RTD was slower in SCI (229.4±123.6 N m s−1) compared with AB (351.6±60.3 N m s−1; P=0.02). Rate for torque relaxation was also slower in SCI (89.6±52.2 N m s−1) versus AB (142.8±16.4 N m s−1; P=0.01).
Effect of joint angle on M-wave amplitude
In both DF and PF muscle groups, the peak-to-peak amplitude of the M-wave was significantly smaller in the SCI group (P<0.05; Table 3). A significant group × angle interaction (P<0.05) in both muscle groups indicated that the M-wave amplitudes did not change significantly with the different joint angles in the SCI group, but tended to increase at the shorter muscle lengths in the AB group. The M-wave area did not change across joint angles in either group.
Effect of joint angle on peak tetanic/MVC torque
The optimal angle for peak-summated force in DF and PF muscles was different between groups (see Figure 2). In the DF muscles, there was a significant group × angle interaction (P<0.01); peak MVC occurred at 10° PF for AB (42.2±5.2 N m), whereas peak TT occurred at 20° PF for SCI (12.1±6.5 N m). In the PF muscles, peak MVC was achieved at 15° DF in the AB group (119.1±61.9 N m); however, there was no significant effect of angle on TT in the SCI group. The twitch:tetanus or twitch:MVC ratio was calculated, revealing a significantly greater ratio (P<0.05) in the SCI versus AB groups in both the DF (0.36 versus 0.09) and PF (0.34 versus 0.19) muscle groups. The DF twitch:tetanus ratio was also found to increase significantly (P<0.05) with increasing muscle length in the SCI group.
Discussion
The results of the present study gave partial support for our first hypothesis that maximum force would occur at shorter muscle lengths in people with SCI. In the PF muscles, the optimal angle for generation of PT was at a shorter muscle length in the SCI group (5° DF versus 15° DF in SCI and AB, respectively). These findings support the earlier work by McDonald et al.,5 in which the torque–angle relationship in the PF muscles was shifted towards plantarflexion in the SCI group, although no optimal angles were identified in that study. In contrast, there was no evidence of any group differences in optimal angle for PT in the DF muscle group; both groups generated PT at the same joint angle of 20° PF. It was more difficult to compare the optimal angles for generation of summated force, as tetanic stimulation was used for the SCI group and MVCs for AB. The significant discomfort associated with 80-Hz stimulation, especially in the PF muscles, led to the decision to use MVCs in the AB group.
The results from this study did not support an SCI-induced transformation to faster fibre types. The RTD was not different between groups in the DF muscles, and was significantly slower in the PF muscles of the SCI group. RTD is dependent on myosin ATPase activity and is directly related to the proportion of type IIb fibres.12 Previous studies have shown that muscles undergo a fibre-type transformation towards a greater representation of type IIb fibres in vastus lateralis1, 13, 14 and soleus muscles2 after SCI. There is some suggestion that the degree of fibre-type transformation may be influenced by the amount of spasticity in a particular muscle after SCI. Previous reports have suggested a preservation of contractile properties and/or an increased expression of slow twitch fibres in muscle that experiences a significant degree of spasticity after SCI.15, 16 Furthermore, Stein et al.17 suggested that if spasticity occurs more frequently in the extensor muscles (such as the soleus), the tibialis anterior muscle would be placed in a stretched position, thus preserving the torque-generating capacity of that muscle. In contrast, the extensor muscles would be exposed to more shortened positions because of both the prolonged sitting position in a wheelchair and the repeated bouts of spasticity. Although this would certainly support the findings of the current study, we did not assess spasticity and cannot attribute the lack of predicted change in contractile speed to levels of spasticity in our subjects. Clearly, the role spasticity has in muscle function and contractile properties after SCI needs further investigation.
The lack of any observable effect of SCI on the magnitude of PT of the DF muscles confirms previous reports,3, 18, 19 and is most likely the result of SCI-induced changes in muscle stiffness. Olsson et al.10 showed a MHC type IIx-dependent increase in muscle stiffness, as measured by passive tension, after SCI. This increase was particularly apparent at long muscle lengths. The significantly greater twitch:tetanus ratio in the SCI group of the present study certainly supports an increase in muscle stiffness, most likely related to a decline in the series elastic component. The increasing twitch:tetanus ratio with increasing ankle angle in the DF of the SCI group lends further support to the contribution of muscle stiffness, as only the twitch torque was found to increase as the muscle was lengthened.
Another noteworthy observation was the inability to generate a muscle twitch in the majority of SCI participants at shortened muscle lengths, particularly in the DF muscle group (see Table 2). Although it is possible that the torques generated were too small to be detected by the force transducer, this may also be an evidence of sarcomere shortening. At extremely short muscle lengths, thick filaments (myosin) collide with Z-lines, inhibiting adequate binding with actin (thin filaments) and thus, decreasing tension development.6 In the SCI group, it seems that this inability to generate torque occurred at even some neutral muscle lengths, which would suggest sarcomere shortening.
The smaller M-wave amplitude noted in both DF and PF muscle groups after SCI is indicative of the vast muscle atrophy and confirms previous reports.2, 3, 17 As M-waves represent the summation of motor unit potentials in response to a maximal stimulus, a reduction in M-wave size is considered as an indirect indicator of muscle atrophy or weakness.20 A smaller M-wave would indicate fewer motor units; therefore, less overall muscle tissue. Smaller M-waves have been correlated with a decreased force-generating capacity in paralyzed thenar muscles.20
The results of this study suggest that when examining changes in muscle properties after SCI, it is important to consider possible changes in the length–tension relationship. Although there seems to be a significant change in PF muscles after SCI, such as a shifted length–tension relationship, decreased PT and M-wave size, there does not seem to be the same magnitude of decrease in DF muscle characteristics. In terms of rehabilitation after SCI, interventions and assisted devices should focus on maintaining or increasing muscle length in muscles that sustain for longer periods in a shortened position. The extended periods of sitting in a wheelchair likely contributes to the shortening (and subsequent loss of force-generating capacity) of the PF muscles, hence it may be prudent to incorporate full leg extension into the daily routine of persons with SCI.
References
Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R . Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 1997; 35: 86–91.
Shields RK . Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol 1995; 73: 2195–2206.
Rodrigues L, Pilutti L, Hicks A . Twitch contractile properties in individuals with spinal cord injury. Appl Physiol Nutr Metab 2007; 32: S76.
Gerrits HL, de Haan A, Hopman M, van der Woude LHV, Jones DA, Sargeant AJ . Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve 1999; 22: 1249–1256.
McDonald MF, Garrison MK, Schmit BD . Length-tension properties of ankle muscles in chronic human spinal cord injury. J Biomech 2005; 38: 2344–2353.
Gordon AM, Huxley AF, Julian FJ . The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 1966; 184: 170–192.
Williams PE, Goldspink G . Connective tissue changes in immobilised muscle. J Anat 1984; 138: 343–350.
Williams PE, Goldspink G . Changes in sarcomere length and physiological properties in immobilized muscle. J Anat 1978; 127: 459–468.
Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G . Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 1972; 224: 231–244.
Olsson MC, Kruger M, Meyer LH, Ahnlund L, Gransberg L, Linke WA et al. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol 2006; 577: 339–352.
Gerrits KH, Maganaris CN, Reeves ND, Sargeant AJ, Jones DA, de Haan A . Influence of knee joint angle on muscle properties of paralyzed and nonparalyzed human knee extensors. Muscle Nerve 2005; 32: 73–80.
Barany M . ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 1967; 50: 197–218.
Castro MJ, Apple DFJ, Staron RS, Campos GER, Dudley GA . Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol 1999; 86: 350–358.
Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D . Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol 2007; 102: 340–349.
Hidler JM, Harvey RL, Rymer WZ . Frequency response characteristics of ankle plantar flexors in humans following spinal cord injury: relation to degree of spasticity. Ann Biomed Eng 2002; 60: 969–981.
Ditor DS, Hamilton S, Tarnopolsky MA, Green HJ, Craven BC, Parise G et al. Na+, K+-ATPase concentration and fiber type distribution after spinal cord injury. Muscle Nerve 2004; 29: 38–45.
Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yang JF, Totosy de Zepetnek J et al. Optimal stimulation of paralyzed muscle after human spinal cord injury. J Appl Physiol 1992; 72: 1393–1400.
Scott WB, Lee SCK, Johnston TE, Binkley J, Binder-Macleod SA . Contractile properties and the force-frequency relationship of paralyzed human quadriceps femoris muscle. Phys Ther 2006; 86: 788–799.
Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 2006; 87: 772–778.
Thomas CK . Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve 1997; 20: 788–799.
Acknowledgements
We thank John Moroz for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelletier, C., Hicks, A. The length–tension relationship of human dorsiflexor and plantarflexor muscles after spinal cord injury. Spinal Cord 48, 202–206 (2010). https://doi.org/10.1038/sc.2009.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.106