Abstract
Study Design:
Cross-sectional study.
Objective:
(1) To quantify intramyocellular lipid (IMCL) content of the soleus muscle. (2) To assess the T2 relaxation rates in the lower extremity skeletal muscles in persons with incomplete spinal cord injury (SCI).
Setting:
Academic Institution, Florida.
Methods:
Eight subjects (42±10 years old; 70±12 kg; 176±10 cm) with chronic (17±9 months post injury) motor SCI (C4-T12; ASIA C or D) and eight matched healthy controls were tested. Localized unsuppressed proton spectroscopy (H-MRS) was performed to estimate total lipid content and individual lipid components; IMCL and extramyocellular lipid (EMCL) from the soleus muscle. T2-weighted imaging of lower extremity muscles yielded muscle T2 rates.
Results:
The IMCL content of the soleus muscle was 3.3 times higher in the patient group as compared to controls (P=0.002; 0.0401 (0.0234–0.0849) versus 0.0123 (0.0090–0.0175)). Similarly, EMCL measures were 4.5 times higher as compared to the controls (P=0.002). Significant differences were observed in the T2 relaxation times of the soleus and gastrocnemius muscles (P<0.05).
Conclusion:
The increased levels of IMCL might interfere with the glucose uptake in skeletal muscle; potentially predisposing persons with incomplete SCI to the development of peripheral insulin resistance. Marked elevations in the T2 relaxation times of the locomotor muscles are reflective of an altered muscle composition.
Similar content being viewed by others
Introduction
Among the 10 000 spinal cord injuries (SCI) that occur annually in the United States, almost 55% are classified as incomplete (www.spinalcord.uab.edu). Similar to patients with complete SCI, individuals with incomplete SCI display a variety of skeletal muscle adaptations including a decrease in muscle cross-sectional area,1 and decrements in voluntary force production and muscle activation.2 Collectively, these musculoskeletal deficits limit the overall functional capabilities of this patient population.1, 2 Furthermore, a relatively sedentary lifestyle following the neurological injury makes persons with incomplete SCI extremely vulnerable to metabolic risk factors. Glucose intolerance and insulin resistance are purported as two of the major metabolic risk factors in the development of cardiovascular complications after SCI.3
Strong evidence in the literature suggests obvious positive correlations between intramyocellular lipid (IMCL) and insulin resistance in individuals with a relatively sedentary lifestyle including healthy individuals,4 elderly5 and persons with obesity.5, 6 Accordingly, IMCL depots in skeletal muscle may provide an indirect measure of insulin resistance. Given the higher reported incidence of insulin resistance in persons with incomplete SCI7 and a purported relatively inactive lifestyle,8 we hypothesized that individuals with incomplete SCI would demonstrate an elevated IMCL content in skeletal muscle. Although studies have demonstrated elevated lipid depots in the lower extremity muscles of persons with complete and incomplete SCI using magnetic resonance imaging (MRI),9, 10 we are not aware of any study that has specifically investigated alterations in the IMCL content in persons with motor incomplete SCI.
Therefore, the overall objective of this study was to quantify the IMCL and extramyocellular lipid (EMCL) content of the soleus (SOL) muscle in persons with incomplete SCI using non-invasive MRI and magnetic resonance spectroscopy (MRS) measures. In addition, we measured the magnetic resonance (MR) transverse relaxation (T2) rates of multiple lower leg muscles because of their sensitivity to alterations in intramuscular fat11 and the demonstrated enhancement in muscle T2 in animal models of incomplete SCI.12
Methods
Subjects
Eight individuals with chronic (17±9 months post injury) motor incomplete SCI (C4-T12; ASIA C or D) participated in this study. Four subjects used a cane or forearm crutches, whereas four used a powered wheelchair as their primary means of mobility (Table 1). In addition, eight able-bodied persons matched in age, weight, height and gender volunteered to serve as control subjects (mean±s.d.; 42±11 yrs old, 73±13 kg and 174±10 cm). Control subjects were recreationally active, but not engaged in any rigorous exercise program.
Before participating in the study, a written informed consent was obtained from all subjects, as approved by the Institutional Review Board at the University of Florida, Gainesville, FL, USA. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Magnetic resonance measurements
For logistical reasons, proton MRS in the SCI group was performed on six participants and MRI was performed on seven individuals.
Proton magnetic resonance spectroscopy
Magnetic resonance spectroscopic relaxometry was implemented to quantify the IMCL and EMCL components and measure the T2 relaxation properties of SOL muscle without contamination from intramuscular lipid.13
The volume of interest (∼35 mm2) was carefully selected using transaxial T1-weighted images, avoiding visible blood vessels and muscle fascia. The T2 relaxation rate of the SOL muscle was determined by acquiring unsuppressed spectra with four different echo times (13, 30, 60 and 120 ms), a repetition time (TR) of 6000 ms, 4 scans, 512 points and 2500 Hz spectral width. In addition, 32 averages were acquired with a echo time (TE) of 120 ms to estimate the total lipid content and individual lipid components; IMCL and EMCL.
Magnetic resonance imaging
Magnetic resonance imaging was employed to investigate alterations in the T2 relaxation properties of the lower extremity muscles of persons with incomplete SCI. MR images of the self-reported more involved lower leg was acquired in the supine position in a 1.5T-superconducting magnet (GE Medical Systems global headquarters: Waukesha, Wisconsin) using a standard length (20 cm) lower-extremity quadrature coil. Multiple slice, spin-echo trans-axial T2-weighted images were acquired using a TR=2000 ms; TE=26, 52, 78 and 108 ms; FOV=16 cm; slice thickness=7 mm and a matrix of 128 × 256.
Data analysis
Magnetic resonance spectroscopy
First, we determined the total intramuscular lipid content along with IMCL and EMCL ratios. A zero order phase correction was performed on the 120 ms raw spectrum (32 averages) and water and lipid spectral peaks were quantified using an Advanced Magnetic Resonance (AMARES) time-domain-fitting algorithm within jMRUI. Chemical shift positions for relevant spectral lipid peaks (IMCL and EMCL) were obtained from proton spectra data collected in healthy adults from the soleus muscle. Whole lipid and water peaks were identified at 1.5 and 4.7 ppm, respectively. Once the amplitudes for the water and whole lipid peak were calculated, the water peak was manually suppressed during data analysis. The lipid resonance peak was subsequently deconvoluted to estimate IMCL and EMCL at approximately 1.25 and 1.4 ppm using the AMARES method. Thereafter, IMCL and EMCL lipid amplitudes were calculated from their spectral peaks and corrected for T2 relaxation effects. IMCL (85 ms) and EMCL (75 ms) T2 relaxation times measured in this study match lipid T2 relaxation values reported in the literature.14 In concurrence with other studies, the overall lipid, IMCL and EMCL content in our study were expressed as a ratio using the spectral water peak as an internal reference.6
Second, we measured the T2 relaxation time of the SOL muscle independent of lipid, assuming a single exponential decay with respect to the four spectral echo times.
Magnetic resonance imaging
In each subject, T2 maps were created for 8–10 image slices. Regions of interests were carefully selected in the SOL, MG (medial gastrocnemius), lateral gastrocnemius and tibialis anterior muscles, avoiding the fascia and visible blood vessels. Characteristic T2 relaxation times were subsequently calculated assuming a single exponential decay with respect to the four imaging echo times. Using the same procedure, T2 maps and the mean T2 value of the bone marrow was calculated to assess the variability in the T2 relaxation measurement.
Statistical analysis
Levene's test showed unequal variance between the SCI and control groups for both T2 and lipid measures. Accordingly, non-parametric Mann–Whitney tests were used to compare the T2 relaxation times and baseline lipid measures between the SCI and control groups. SPSS for Windows (Version 11.0.1) (SPSS Inc, Chicago, IL, USA) was utilized for all statistical analyses. Alpha level was set at 0.05.
Results
Intramuscular lipid
Persons with incomplete SCI demonstrated significantly higher total lipid, IMCL and EMCL levels in the SOL muscle as compared to healthy controls. The total lipid content normalized to water was on an average 3.2 times higher in the subjects with incomplete SCI as compared to controls (Figure 1). The IMCL content of the SOL muscle was 3.3 times higher in the patient group as compared to controls (P=0.002; median (minimum–maximum) 0.0401 (0.0234–0.0849) versus 0.0123 (0.0090–0.0175)). Similarly, EMCL measures were 4.5 times higher as compared to the controls (P=0.002; median (minimum-maximum) 0.0578 (0.0272–0.0771) versus 0.0129 (0.0079–0.0228)). Note that the subjects with incomplete SCI also showed a much wider distribution of values for all lipid ratios as compared to the controls (Figure 1). Finally, individual data revealed that every person, ambulatory and non-ambulatory, with incomplete SCI showed an elevation in the overall lipid, IMCL and EMCL measures as compared to their corresponding controls (Figures 2a and b).
Boxplot with median and percentile values depicting variability in EMCL/water, IMCL/water and total soleus muscle lipid/water ratios in the SCI and control groups. Note the large variability in the SCI (hatched bars) versus control group for all ratios. The EMCL/water ratio shows an extreme value from subject S6 (O). EMCL, extramyocellular lipid; IMCL, intramyocellular lipid; SCI, spinal cord injury.
(a) Comparisons of IMCL to water ratio (IMCL/water) for individuals with incomplete SCI and their corresponding controls (subjects S1 and S4 are ambulatory). (b) Individual data comparisons of EMCL to water ratio (EMCL/water) between persons with incomplete SCI and corresponding controls (subjects S1 and S4 are ambulatory). EMCL, extramyocellular lipid; IMCL, intramyocellular lipid; SCI, spinal cord injury.
Muscle T2 relaxation times
Representative T2-weighted axial images of the lower leg of healthy controls, ambulatory and non-ambulatory incomplete SCI subjects are shown in Figure 3. Overall, persons with incomplete SCI showed 11–26% higher T2 relaxation values in the tested muscles (Table 2). Significant differences were observed in the T2 relaxation times of the SOL (P=0.011, 11%) and MG (P=0.005; 26%) muscles. Interestingly, all persons with incomplete SCI showed higher T2 values of the MG and SOL muscles obtained by MRI compared to the control subjects. In contrast, the T2 relaxation time of bone marrow was consistent in the SCI and control subjects (1% difference).
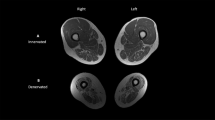
Representative T2-weighted transaxial proton magnetic resonance images of the lower leg in (a) ambulatory individual with incomplete SCI (S1), (b) non-ambulatory individual with incomplete SCI (S8) and (c) able-bodied control subject (C8). A, anterior; L, lateral; LG, lateral gastrocnemius; M, medial; MG, medial gastrocnemius; P, posterior; SCI, spinal cord injury; SOL, soleus, TA, tibialis anterior.
Calculations of SOL muscle T2 relaxation times by spectroscopy coincided with T2 measurements from MRI. Patients with an incomplete SCI showed a significant (14%, P=0.017) increase in the baseline T2 relaxation times of the SOL muscle as compared to the control group (median (minimum–maximum) 35.8 ms (30.8–41.5 ms) versus 31.1 ms (29.1–31.4 ms)).
Discussion
This study utilized localized proton spectroscopy and T2-weighted imaging to characterize skeletal muscle in persons with chronic, motor incomplete SCI. Using image-guided volume localized proton spectroscopy we quantified intramuscular lipid in the predominantly slow twitch SOL muscle. Interestingly, we found a marked increase in the intramuscular lipid content of all individuals with SCI compared to controls. This phenomenon of elevated muscle lipid has been reported in a number of clinical populations including individuals with stroke15 as well as both complete9 and incomplete SCI.10 Elder and co-workers demonstrated that persons with complete SCI display an almost fourfold increase in intramuscular fat relative to controls, and that these values correlate with plasma glucose levels during an oral glucose tolerance test (r2=0.52 and 0.71, P<0.001). In another study, a threefold elevation in intramuscular fat was observed in persons with chronic motor incomplete SCI.10 Previous studies in SCI patients, however, did not distinguish between IMCL and EMCL, but instead used MRI to estimate total intramuscular lipid content. An advantage of the present study is that using localized H-spectroscopy we were able to individually quantify both IMCL and EMCL.
Our findings demonstrate significant elevations in the ratios of IMCL and EMCL to water following incomplete SCI. Moreover, the IMCL and EMCL content measured in the SOL muscle (relative to the water peak) of control subjects in our study fell within the range of those reported previously.4 Although IMCL is purportedly influenced by diet and exercise; studies also report strong correlations between IMCL and insulin resistance irrespective of diet, age, weight, activity level and gender.5 Nevertheless, in our study, while gender, body weight, physical activity and age were well controlled, elevations in myocellular lipids (3.3 (IMCL) and 4.5 (EMCL) times elevation relative to controls) are far greater than those attributable to dietary influences (0.32 times elevation in previous dietary studies).4 The highly elevated IMCL levels in the incomplete SCI group in this study raises concern because of their reported association with insulin resistance in sedentary individuals with normal body weights as well as obese individuals.6 Moreover, skeletal muscle is the major depot (∼80%) for blood glucose and marked muscle atrophy has been shown to be a secondary risk factor for insulin resistance.5 The combination of muscle atrophy,1 relative inactivity8 and high IMCL levels in persons with incomplete SCI potentially renders this patient population particularly vulnerable to altered glucose homeostasis. Accordingly, in the light of recognizing elevated IMCL content as detected by MR measures, our findings suggest that such metabolic adaptations should be assessed in response to the injury as well as different rehabilitation protocols in this patient cohort.
In addition to measuring the IMCL, we also studied the T2 relaxation characteristics of the lower extremity muscles in persons with chronic SCI. Using MRI, we found 11–26% higher T2 values in the lower extremity muscles of persons with incomplete SCI, with maximum relative changes in the MG muscle. Owing to partial volume filling, estimates of muscle T2 using MRI are influenced by both water and lipid protons within the muscle. Lipid has an inherently longer T2 relaxation time (85–90 ms) than muscle (T2 equal to 31–33 ms) at 1.5T; therefore, it is not surprising that persons with incomplete SCI, who show higher amounts of intramuscular lipid, would display increased T2 relaxation times on MRI. However, using proton spectroscopy we also assessed the T2 relaxation time of the SOL muscle independent of contributions of lipid, and found that the muscle T2 in persons with incomplete SCI remained elevated.
Elevated muscle T2 relaxation times observed in our study are in concurrence with data acquired in animal models of incomplete SCI. Liu et al.12 showed significant elevation in the T2 relaxation times of rat hindlimb muscles after 1 week of spinal contusion injury, with maximum changes in the slow SOL muscle. Furthermore, elevated muscle T2 times have also been reported in both human16 and animal models of peripheral denervation.17 Although exact mechanisms of the enhanced T2 relaxation times in these models remain unknown, these studies speculate that the elevated T2 relaxation times are either due to an increase in extramyocellular fluid12 or due to capillary enlargement and increased muscular blood volume.16, 17 Indeed, Liu and co-workers showed an enhancement in extramyocellular space in the paralyzed rat skeletal muscle after incomplete SCI during concomitant qualitative histological assessment. Interestingly, in our study we observed the highest muscle T2 values in wheelchair users (S5, S6, S7 and S8). As persons with motor incomplete SCI have vascular disturbances including venous vascular dysfunction,18 a continuous dependent position of their lower extremities (in the seated position) most likely leads to accumulation of myocellular water in the postural leg muscles.
Alternatively, elevated T2 values have been reported in several models of muscle injury including reloading injury,19 muscle degeneration13 and contraction-induced injury.20 Moreover, based on changes in muscle T2 after isometric contractions, previous studies have suggested an increased susceptibility to muscle injury after complete SCI in humans.20 Factors including unloading and inactivation of affected muscle as well as skeletal muscle atrophy seemingly contribute to this phenomenon. Although persons with incomplete SCI have partial sparing of the spinal cord, relative unloading and inactivation of their lower limb muscles might predispose skeletal muscle to injury. As such, the elevated baseline T2 values from our data might be reflective of damaged muscle. Clearly, future studies using advanced imaging and spectroscopic measurements are warranted to identify the cause of elevated T2 relaxation times following incomplete SCI.
We recognize potential limitations of our study. First, the sample size of our incomplete SCI group limits conclusions concerning the impact of injury level, duration and/or severity of injury on myocellular lipid levels. As incomplete SCI reflects a very heterogeneous population with varying ambulatory status, future studies on a larger sample size might add value. Secondly, we did not test glucose tolerance or insulin sensitivity of our subjects; thereby limiting our ability to directly associate the myocellular lipids with the development of insulin resistance. Future studies on persons with incomplete SCI are warranted to confirm the association of IMCL accumulation and development of insulin resistance. Nevertheless, based on extensive literature that correlates IMCL content with development of insulin resistance, we believe our data yields valuable information concerning potential risks associated with incomplete SCI and provides a foundation for future studies.
In conclusion, persons with incomplete SCI demonstrated an increase in the total lipid, IMCL and EMCL content of the SOL muscle and enhancements in the T2 relaxation properties of the lower leg muscles. Future studies are necessary to identify the relationships between IMCL and insulin resistance along with the functional and physiological impacts of increased IMCL in this population.
References
Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 2006; 87: 772–778.
Jayaraman A, Gregory CM, Bowden M, Stevens JE, Shah P, Behrman AL et al. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord 2006; 44: 680–687.
Jacobs PL, Nash MS . Exercise recommendations for individuals with spinal cord injury. Sports Med 2004; 34: 727–751.
Stettler R, Ith M, Acheson KJ, Decombaz J, Boesch C, Tappy L et al. Interaction between dietary lipids and physical inactivity on insulin sensitivity and on intramyocellular lipids in healthy men. Diabetes Care 2005; 28: 1404–1409.
Goodpaster BH, Wolf D . Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes 2004; 5: 219–226.
Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002; 51: 1022–1027.
Bauman WA, Spungen AM . Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001; 24: 266–277.
Ditunno Jr JF, Burns AS, Marino RJ . Neurological and functional capacity outcome measures: essential to spinal cord injury clinical trials. J Rehabil Res Dev 2005; 42: 35–41.
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA . Intramuscular fat and glucose tolerance after spinal cord injury—a cross-sectional study. Spinal Cord 2004; 42: 711–716.
Gorgey AS, Dudley GA . Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007; 45: 304–309.
Huang Y, Majumdar S, Genant HK, Chan WP, Sharma KR, Yu P et al. Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging 1994; 4: 59–64.
Liu M, Bose P, Walter GA, Anderson DK, Thompson FJ, Vandenborne K . Changes in muscle T2 relaxation properties following spinal cord injury and locomotor training. Euro J Appl Physiol 2006; 97: 355–361.
Walter G, Cordier L, Bloy D, Sweeney HL . Noninvasive monitoring of gene correction in dystrophic muscle. Magn Reson Med 2005; 54: 1369–1376.
Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol 1999; 276: E977–E989.
Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF . Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 2002; 83: 1703–1707.
Koltzenburg M, Bendszus M . Imaging of peripheral nerve lesions. Curr Opin Neurol 2004; 17: 621–626.
Wessig C, Koltzenburg M, Reiners K, Solymosi L, Bendszus M . Muscle magnetic resonance imaging of denervation and reinnervation: correlation with electrophysiology and histology. Exp Neurol 2004; 185: 254–261.
Hopman MT, Nommensen E, van Asten WN, Oeseburg B, Binkhorst RA . Properties of the venous vascular system in the lower extremities of individuals with paraplegia. Paraplegia 1994; 32: 810–816.
Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K . Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve 2005; 32: 605–612.
Bickel CS, Slade JM, Dudley GA . Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol 2004; 91: 308–313.
Acknowledgements
Sponsorship: Supported by the National Institutes of Health (Grant number NIH-RO1HD037645 and NIH-KO1HD01348).
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part to the American Physical Therapy Association, February 2007, Boston, MA, USA.
Rights and permissions
About this article
Cite this article
Shah, P., Gregory, C., Stevens, J. et al. Non-invasive assessment of lower extremity muscle composition after incomplete spinal cord injury. Spinal Cord 46, 565–570 (2008). https://doi.org/10.1038/sc.2008.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.10