Abstract
Reactive primary and secondary minerals play a critical role in the transformation and stabilization of organic matter (OM) in soil, a critical aspect that has been largely overlooked in existing literature. In this regard, we propose a new model known as the “reactive mineral sink” (RMS) to illustrate three primary mechanisms through which these minerals drive the bioprocessing, transformation, transport and stabilization of OM in soil. Firstly, from a biological perspective, reactive minerals influence enzymatic and microbial OM processing through binding enzymatic proteins or influencing the structure of microbial communities. Secondly, from a chemical standpoint, these minerals have the capacity to adsorb OM and/or coprecipitate with it, leading to a more diverse distribution of OM in the soil. This distribution, in turn, triggers OM transformation through chemical catalysis and redox reactions. Thirdly, on a physical level, reactive minerals have a substantial impact on soil architecture, aggregate dynamics, porosity development, and hydrological processes. These physical changes then affect the transport, reprocessing and stabilization of OM. The RMS model provides a conceptual framework that underscores the fundamental role of reactive minerals in driving the dynamics of OM and carbon (C) sequestration in natural soil. Furthermore, it promotes the restoration of soil biogeochemical processes and ecological resilience. We advocate for the implementation of strategies based on the RMS model to enhance the sequestration of organic C in soils for the purposes of rejuvenating soil health and mitigating CO2 emission.
Similar content being viewed by others
Introduction
Soil organic matter (OM) comprises a wide array of organic compounds within the soil that undergo progressive decomposition1. It includes organic material from plant and animal sources, their decomposition products, as well as biomass and metabolic products of soil microorganisms. The formation and stabilization of OM are critical to establishing a resilient soil structure and ensuring optimal biogeochemical functionality2. In the context of mitigating global climate change, soil OM serves as the predominant carbon (C) reservoir within terrestrial ecosystem, and its long-term stability is governed by its physical and chemical properties1,3. In the conventional view, soil OM consisted primarily of recalcitrant lignin-rich compounds derived from plant biomass1. However, it later became evident that microbial-derived OM, formed within the soil profile itself, also contributes significantly to the stable pool of soil OM. To emphasize the role of soil microbes in the decomposition of OM and the creation of new forms of microbial-derived OM, the concept of the “microbial carbon pump” (MCP)4 was introduced. More recently, the protection of OM by soil minerals gained recognition as a crucial mechanism for the storage of OM over the long term5. Consequently, numerous studies have focused on the adsorption of OM compounds to a variety of soil minerals, ranging from ferric oxyhydroxides to clay minerals6,7. The critical roles of minerals in OM persistence and accumulation in soils were further introduced in the “soil mineral carbon pump” model proposed by Xiao et al.8.
Nevertheless, critical knowledge gaps still need to be addressed concerning the types of minerals involved in stabilizing OM, as well as biological, chemical and physical roles of soil minerals in OM processing, transformation and stabilization. Indeed, as suggested by Kleber et al.9, the adsorption of OM onto mineral surfaces merely represents the initial phase of organo-mineral interactions. Subsequently, a series of additional reactions take place, encompassing mineral-catalyzed OM transformations, the generation of reactive oxygen species (ROS) induced by minerals, and the oxidation of OM. Previous studies10,11,12 have highlighted mineral-mediated biological processes, such as mineral-enzyme-mediated OM transformation10. Reactive minerals in soil can also shape soil microbial communities and activities11,12, thus influencing microbial OM processing. Furthermore, minerals act as cementing agents, crucial in binding and preserving soil aggregate structure, thereby governing the storage and stabilization of OM13,14. From these, we propose a greater focus on the pivotal roles of reactive minerals in regulating OM dynamics, since minerals are enduring substrates within soil.
To facilitate a clear and comprehensive discussion, we propose a conceptual framework, namely the “reactive mineral sink” (RMS) to illustrate plausible pathways of soil reactive minerals in OM biogeochemistry. The RMS can be defined as a matrix composed of a variety of minerals characterized by a high density of surface charges and/or significant thermodynamic potential. These minerals encompass both less stable primary minerals (e.g., biotite, amphibole) and relatively stable secondary minerals, such as smectite, montmorillonite, vermiculite, kaolinite, as well as amorphous minerals like Fe(III)/Mn(IV)/Al(III) oxyhydroxides (as listed in Table 1).
We evaluate mineral stability using the “Gibbs free energy of formation of minerals”. This parameter governs the phase equilibria of mineral systems and serves as the basis for their geochemical behavior in soil, including processes such as mineral weathering and transformation15. Minerals with higher Gibbs free energy of formation, such as primary minerals, typically exhibit lower stability and are more prone to weathering, leading to the formation of secondary minerals16,17.
The RMS is expected to systematically influence the character and stabilization of OM through multiple pathways. It may impact OM composition by regulating microbial OM processing, resynthesis, and chemical reactions, ultimately leading to the creation of persistent OM. Additionally, the RMS drives OM diversity and its stability within minerals and/or soil aggregates by influencing the physical transport of OM and interactions between OM and minerals. The functional significance of RMS is paramount in augmenting the storage of soil OM, bolstering biogeochemical processes, promoting soil health, and fostering the development of soil resilience and sustainable productivity.
RMS drives OM dynamics in soils
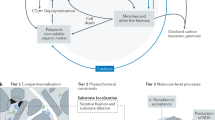
Reactive minerals in soils play a central role within the soil ecosystem. They function as a dynamic hub, driving the bioprocessing, transformation, transport, and stabilization of OM via an interplay of biological, chemical, and physical processes, as illustrated in Fig. 1.
(i) Biological processes: The reactive minerals can directly bind exoenzymes and influence enzymatic OM decomposition and transformation. The mineral matrix can also create micron-sized niches that promote different microbial communities that, in turn, can alter OM decomposition, reprocessing and resynthesis. (ii) Chemical processes: The reactive mineral matrix causes heterogeneous OM distribution and molecular transformation because different mineral surfaces have different chemical reactivities that will impact OM adsorption, chemical reactions and subsequent organo-mineral association. (iii) Physical processes: Mineral coagulation and dispersion influence aggregate formation and turnover, leading to OM protection and/or liberation. The mineral matrix can also influence hydrological processes that regulate the transport of OM, microbes, colloids and solutes through the matrix, resulting in OM relocation, reprocessing and stabilization.
Biological processes
The RMS influences soil OM biodegradation and resynthesis by driving biological processes in the following ways:
(1) Reactive minerals exert their influence on extracellular enzyme, commonly referred to as exoenzymes21, by binding to enzyme proteins. This interaction subsequently affects the extracellular pathway of OM decomposition and modification10, resulting in alterations to OM chemistry and its stability. Exoenzymes, being proteins rich in nitrogen-containing moieties, are well known for their strong tendency to bind with a variety of minerals22. When associated with minerals, functional groups within enzymes may undergo modifications10,22, which can impact their catalytic capacity at transforming OM. Furthermore, certain reactive minerals, including Fe(II) minerals, can trigger the production of ROS23, which in turn, influence exoenzyme activities and thereby regulate their role in OM transformation.
(2) Reactive minerals create microenvironments and pore spaces through compartmentalization, providing localized habitats with diverse water, nutrient, and oxygen conditions for microorganisms. The assemblage of minerals and the formation of aggregates contribute to the generation of these pore spaces. Pore spaces within aggregates and interstitial spaces between aggregates (typically measuring tens of micrometers in diameter) are essential microhabitats for soil microorganisms24,25. This spatial heterogeneity can lead to diversity within soil microbial communities and exerts control over OM decomposition and reprocessing via microbial catabolism/anabolism, often referred to as the “microbial carbon pump”4. Pores within the size range of 30–90 μm have been identified as playing significant roles in the protection and/or mineralization of OM25. The mineral phases, composition, and characteristics directly impact porosity structure and pore sizes, which, in turn, regulates microbial OM processes.
(3) Reactive minerals have the capacity to alter microbial community structure by preferentially favouring the development of specific microbial communities in the “mineralosphere”12. Minerals play a significant role in shaping microbial communities and their activities. They provide microbial habitats on mineral surfaces, essential nutrients, energy sources (e.g., during Fe redox reactions), and can also introduce potentially harmful elements like heavy metals and ROS11,23. Changes in microbial community structure and functions have consequences for the pathways for microbial OM decomposition and regeneration4. For instance, microorganisms in the mineralosphere can utilize low-molecular-weight organic acids and monosaccharides during mineral weathering26, potentially leading to increased production and accumulation of microbial metabolites and/or necromass4. Different types of minerals have distinct effects on microbial OM processes and molecular composition. For example, a study demonstrated that kaolinite and montmorillonite had differing impacts on microbial communities, resulting in differences in the molecular composition of OM27.
Reactive minerals can influence microbial activities and metabolisms, transforming OM. For instance, minerals rich in Fe(II) can stimulate the activities of Fe(II) oxidizing bacteria, especially heterotrophic/mixotrophic Fe(II) oxidizers such as nitrate-reducing Fe(II) oxidizers, which utilize OM as an energy source28. Fe(II)-rich reactive minerals can also induce the production of ROS, selectively inhibiting specific microorganisms by causing oxidative damage to lipids and proteins29,30. In addition, minerals rich in Fe(III) can facilitate Fe(III)-reducing bacteria that metabolize by oxidizing simple organic compounds and discarding electrons onto the Fe(III)-bearing minerals, while producing dissolved Fe(II) and bicarbonate31,32.
Soils in the early stages of pedogenesis, including Cambisols and post mine tailing soils33, have experienced limited soil development and retain a substantial amount of less stable primary minerals. These minerals undergo weathering driven by both biological and non-biological factors, resulting in significant mineral changes and subsequent mineral-mediated bioprocessing of OM. Notably, when minerals undergo congruent dissolution, all constituent elements are dissolved34. These elements released from the minerals can provide essential nutrients for soil microbes, thus facilitating microbial growth and the decomposition/ reprocessing of OM34.
Furthermore, the mineralogical composition can influence the dynamics of OM through the regulation of plant root activities. For instance, Liang et al. (2023)35 demonstrated that minerals and roots interactively influence the stabilization and loss of OM. Under conditions where low-reactivity minerals (e.g., kaolinite) exist, roots enhanced OM mineralization and reduced the association of OM with minerals. Conversely, when highly reactive minerals (e.g., ferric oxyhydroxides) are present, root exudates promoted greater association of OM with minerals. Moreover, a recent study36 revealed that minerals, such as kaolinite and ferrihydrite, resulted in significant differences in the organic molecular composition, particularly lipids, in rhizosphere soil.
Chemical processes
The RMS exerts its influence on the distribution patterns and molecular properties of OM through adsorption and mineral-mediated chemical reactions. OM compounds can be adsorbed onto mineral surfaces through mechanisms such as ligand exchange, hydrogen bonding, cation bridging, and hydrophobic interactions37. The heterogeneous distribution of OM arises from the presence of mixed minerals with varying affinities and capacities for the adsorption and stabilization of OM. Minerals that have a higher specific surface area and a greater density of surface charges can stabilize a greater number of OM compounds compared to minerals with lower surface area and reactivity. These properties lead to enrichment of OM in specific minerals within the soil9,37. For example, it has been shown that OM compounds in soil aggregates are primarily found in a heterogeneous pattern, often associated with Al/Fe rich phyllosilicates rather than quartz at the submicrometer scale38. In paddy soil, an increase in ferric oxyhydroxide content from 13.7 to 55.8 g kg−1 Fe resulted in an enhanced stabilization of OM, affirming the pivotal role of ferric oxyhydroxides in preserving soil OM. The impact of soil minerals on OM stabilization is contingent on the properties of the minerals and the characteristics of OM39. In a recent study40, it was found that hematite-rich tailings exhibited a greater capacity to stabilize OM during early pedogenesis in comparison to biotite-rich tailings40. Additionally, vermiculites, in contrast to kaolinite and illite, demonstrated a propensity for promoting the preservation of plant litter-derived carbohydrates through organo-mineral associations. This phenomenon is likely attributed to the higher specific surface area and stronger affinity for carbohydrates that vermiculites possess relative to kaolinite and illite41.
In addition to adsorbing OM, reactive minerals or their derived ions can also coprecipitate with organic molecules, giving rise to organo-mineral associations. The size of these associations can vary, spanning from nano- to micro-scales, which results from interactions between inorganic oligomers and organic compounds. For example, soluble ions derived from biotite minerals, such as Fe, Si, and Al, have been observed to co-precipitate with organic ligands, forming loosely bounded organo-mineral associations42. These organo-mineral associations, including coprecipitates, play a vital role in protecting OM from mineralization in soils. For example, the coprecipitation of ferrihydrite with OM has been shown to effectively inhibit OM mineralization in anoxic soils43,44. This protective effect is attributed to the capacity of organo-mineral coprecipitates to function as essential cementing agents or nuclei for formation of soil aggregates. This process acts as a shield, safeguarding OM from microbial mineralization45.
Minerals can facilitate the transformation of OM through multiple processes, including electrolytic and/or hydrolytic breakdown of macromolecules, heterogeneous oxidation, and direct oxidation mediated by transition elements (e.g., Fe or Mn)9. Some minerals, such as Mn(IV) oxides, sulfides, and magnetite may induce hydrolytic breakdown of large macromolecular organic molecules into small molecules46. Some nanosized mineral particles exhibit intrinsic enzyme-like activities akin to those derived from biological sources, effectively catalyzing the transformation of OM47. For instance, a recent study revealed that magnetite nanoparticles possess an intrinsic enzyme-like activity that is similar to that of natural peroxidases, facilitating the oxidation of OM47. These nanominerals with enzyme-like characteristics are called “nanozymes”48. In another study, it was observed that ferrihydrite and/or birnessite displayed efficient catalytic activity in promoting Maillard reactions. This catalysis led to the geopolymerization of small and simple molecules, resulting in the formation of complex and more recalcitrant compounds49.
Redox sensitive minerals, such as biotite, magnetite, Fe(II) sulfides, and Mn/Ti oxides, can efficiently catalyze the transformation of OM into form new molecules9. In floodplain soils, it was discovered that the primary driving force behind the oxidation and preservation of OM was reactive Fe(II), surpassing microbial activity50. Ferrous iron-rich minerals have the ability to induce the production of ROS, leading to the oxidation of OM51. ROS, including hydroxyl radical (HO•) and hydrogen peroxide (H2O2), can enhance the mineralization and oxidative transformation of OM. This, in turn, leads to an increase in the biochemical stability of OM and the formation of organo-mineral complexes that can contribute to the long-term stabilization of OM9,52. The interactions between OM and ROS can further lead to the oxidation of complex OM, resulting in the production of low molecular weight organic compounds characterized by an abundance of carboxyl groups. These compounds readily associate with minerals, contributing to organo-mineral associations52,53. Additionally, certain fungi-mediated redox reactions have been observed to facilitate both the decomposition of OM and the oxidative preservation of mineral-stabilized OM on fungal surfaces54.
ROS is generated through reactions between water and O2, primarily occurring at defect sites on minerals containing Fe(II). Furthermore, ROS production can occur as a result of photochemical electron transfer, especially involving minerals like Fe(II)-bearing phyllosilicates (e.g., biotite, chlorite) and Fe(III) oxides (e.g., hematite)55,56. It is well-documented that HO• can be created through Fenton oxidation, which involves a catalytic reaction chain incorporating H2O2, Fe(II), and ferric oxyhydroxides52. ROS stimulate OM mineralization and promote long-term C stabilization by enhancing the resilience of residual OM and encouraging the formation of organo-mineral complexes52. Studies33,57 have also found that mineral-associated OM was enriched in oxidized compounds, such as carboxyl groups, in soils with different mineralogical compositions. Moreover, electron transfer processes occurring during mineral-mineral interactions in soil can lead to the oxidation or reduction of OM compounds. For instance, electron transfer from Fe(II)-bearing phyllosilicates, such as nontronite, to ferric oxyhydroxides, like ferrihydrite, may induce the reduction of OM compounds during their association with these minerals58.
The weathering of poorly stable primary minerals in soils typically results in the development of surface-reactive secondary minerals and the accumulation of poorly crystalline minerals. These secondary minerals often possess high specific surface areas and variable charge characteristics, rendering them suitable for the binding and stabilization of OM37. For example, our research59 found that the mineral weathering processes in early soils enriched with biotite could generate secondary ferrihydrite and/or Fe(III)-Si rich amorphous minerals with elevated affinity for OM. This leads to shifts in the chemical diversity and composition of dissolved OM59. Additionally, mineral weathering processes result in the release of cations like Ca2+, Mg2+ and Fe3+ into the soil solution. These cations can then form complexes with organic compounds37. Because of the complexity and dynamic changes in mineralogical composition and properties, as well as the molecular profiles of soil OM, it is crucial to acknowledge that the strength of organo-mineral associations is influenced by the combined effects of minerals within the matrix, rather than examining individual isolated mineral phases. This consideration should be a central focus in future studies.
Physical processes
The reactive mineral matrix in soil can encompass a variety of minerals that can aggregate together with OM to create soil aggregates. Within these aggregates, OM becomes occluded and thus safeguarded, resulting in low bioaccessibility13. Key factors in this process include secondary phyllosilicate minerals, such as smectite, and amorphous minerals, such as short-range ordered Fe/Si minerals, which function as mineral cements that maintain aggregate stability and protect OM in the forms of both occluded particulate OM and mineral-associated OM within the aggregates13. The assembly of minerals and the formation of soil aggregates can further produce pore spaces and provide localized niches both inside and outside the aggregates with varying environmental conditions (oxygen, water, nutrient) for microbial colonization24 and microbial processing of OM. In addition to protecting OM in soil aggregates, certain minerals like clay, calcite, and metal oxides have been reported as having the capacity to encase OM, such as humic substances, sugars, and amino acids, within their developing crystalline formations60. In a similar manner, authigenic minerals precipitated by bacteria can completely encase the cells, thus enhancing their preservation potential61,62.
It is well-recognized that soil aggregates themselves cannot represent the entire soil structure underpinning its functionality. As proposed by Vogel et al. (2022)63, soil architecture resulting from soil aggregation, which includes the spatial arrangement of pore networks as well as various minerals and organic components, is critical to soil biogeochemical functions. Properties of soil reactive minerals, such as surface charge, surface area, particle size, crystallinity and covalent reactivity, contribute to the soil architecture and hierarchical pore networks. These factors ultimately regulate hydrological processes64, including the transport of OM, colloids and microbes together with water flow65. Biological and chemical weathering processes could bring about changes to mineral composition that influence soil hydrological processes and the transport of OM. As a result, some OM can be vertically transported from topsoil into subsoil66. These latter processes create feedback loops influencing the biological and chemical processes described above for the transformation and stabilization of OM under fluctuating environmental conditions. For example, in grassland soil (Eutric Fluvisol), there was a decrease in low-molecular weight compounds, while larger microbial-derived molecules increased in the subsoil compared to topsoil66.
Multi-process interactions
RMS-mediated biological, chemical, and physical processes occur simultaneously in soil and collectively determine the composition, distribution, and stabilization of OM in soil. For instance, mineral assemblage and soil aggregate formation (physical processes) can both occlude OM and provide microporosity within aggregates. These, in turn, exhibit variable environmental conditions (i.e., redox, water, salts, nutrient etc) which give rise to the development of specific microbial communities67 and associated microbial OM processing (biological processes). Mineral phases within aggregates can be shielded from interacting with OM compounds in the pore spaces between aggregates, thereby reducing OM transformation processes (chemical processes). Direct reactions between redox sensitive minerals and OM can also lead to alterations in mineral composition and surface properties68, causingchanges to mineral matrix-driven physical and biological processes. Furthermore, reactive minerals can influence pore spaces amongst aggregates of different sizes, leading to a steep gradient of oxygen and nutrient availability. This, in turn, influences the soil food web at micrometer scale and activities of microbial communities within aerobic and anaerobic niches, resulting in diverse microbial OM processing24 (biological processes).
Some OM can be vertically transported from the topsoil into the subsoil via water flow (physical processes). Subsoil properties, such as mineralogical composition, oxygen availability, temperature, OM content, and microbial abundance and diversity, differ from those of the topsoil. These distinctions collectively influence microbial-mediated OM processes (biological processes) in the two soil layers66. However, due to the presence of reducing conditions, the presence of transition elements in the subsoil, such as Fe(II), can actively mediate chemical reactions for OM oxidation50 (chemical processes). This underscores the critical role of the mineral matrix in driving chemical changes in OM within the subsoil, highlighting the significance of these processes in soil OM dynamics.
Implications of RMS driven soil OM dynamics
The RMS model offers a comprehensive framework for understanding the dynamics of OM mediated by a changing reactive mineral matrix and suggests sustainable strategies for fostering soil ecological restoration, enhancing soil resilience, and facilitating carbon sequestration. The quantity and quality of soil OM underpin soil physical structure, its hydrological conditions, chemical stability, as well as various biogeochemical processes such as nutrient cycling, microbial community and functions69. Consequently, the dynamics of OM driven by RMS are critical for preserving and restoring the physical structure and biogeochemical functionality of degraded soils resulting from poor land management and mining impacts33,70.
It is important to emphasize that the dynamics of OM driven by RMS plays a crucial role in regulating and improving soil health. According to Lehmann et al.71, soil health is defined as “the capacity of soil to function as a vital living ecosystem that sustains plants, animals and humans and connects agriculture and soil science to policy, stakeholder needs and sustainable supply-chain management.” When conducting a comprehensive assessment of soil health, a range of physical, chemical, and biological indicators are used to evaluate the overall condition of the soil71,72. Generally, physical indicators include soil aggregate stability, water content, and bulk density; chemical indicators involve factors such as pH, extractable N, P, K, and other essential elements such as Fe, Mg, Mn and Zn; while biological indicators include soil organic matter fractions, labile carbon content, soil protein content, and respiration. Therefore, a comprehensive soil health index should be constructed by considering the significance and interplay of these physical, chemical, and biological indicators, all of which collectively contribute to the assessment and management of soil health71.
Reactive minerals and their interactions with OM can contribute to soil health through various pathways: (1) They can impact the forms and partitioning of OM via organo-mineral interactions, leading to changes in OM fractions (such as labile or persistent OM, mineral unprotected or mineral-associated OM). These changes serve as key indicators of soil health71,72. (2) These minerals play a significant role in enhancing soil aggregate stability, which is an important indicator of soil health72. Minerals and/or organo-mineral association are essential components and cementing agents for soil aggregation. (3) They have the potential to influence the availability of essential nutrients like N, P, K, Ca, Mg and Fe, thereby regulating plant growth—one of the key ecosystem services considered in comprehensive assessments of soil health71. (4) Additionally, they participate in retaining pollutants, such as heavy metals and organic toxins, by stabilizing them with minerals. This retention helps reduce the leaching of pollutants from soil into water sources. This enhances water quality, another crucial ecosystem service assessed in soil health71.
Furthermore, RMS provides a new framework for estimating and enhancing soil organic carbon (OC) sequestration in soils with different mineralogy. This framework holds significant in augmenting soil OM stocks as part of climate change mitigation efforts. The existing C models (e.g., RothC, Socrates, SOMM, NCSOIL, and DNDC) emphasize the biochemical persistence of OM compounds73, often overlooking the intrinsic influence of soil mineral composition, mineral phase and energy dynamics in regulating OM chemistry and stabilization9. While some models, such as CENTURY and DAISY, consider the role of clay minerals in OM stabilization through adsorption processes, they do not account for the comprehensive range of processes mediated by reactive minerals, as described in the RMS model. For instance, in the RMS model, it is demonstrated that “labile” plant derived OM can transform into “persistent” OM after undergoing adsorption and mineral-driven transformations74. Consequently, for accurate estimates of terrestrial soil C sequestration, modeling should consider the parameters of the reactive mineral matrix within a soil, including mineral composition, abundance, and phases, as well as surface reactivity characteristics such as charge and redox potential.
RMS-based strategy for enhancing soil OC sequestration
The multiple functionalities of reactive minerals in soil OM dynamics give rise to their roles in mitigating and managing soil OC sequestration and stabilization. Here, soil OC refers specifically to the carbon component of soil OM. It is a critical component in understanding soil’s role in carbon sequestration for climate change mitigation, as well as soil conservation practices. The knowledge of RMS driven OM dynamics provides a fundamental basis for the development of RMS based strategies towards increasing the capacity of OC sequestration and storage in terrestrial soil.
Soil OC fractions
Soil OC is sequestered through a combination of physical and chemical protection within particulate OM (POM) and mineral-associated OM (MAOM)75. POM is primarilty composed of OM derived from plant litter and partially decomposed plant tissues, whereas MAOM is predominantly composed of OM originating from microbial biomass and their metabolites intimately bound to minerals22. MAOM, due to its mineral protection, is more persistent than POM75. POM can be progressively decomposed by microbes, giving rise to a broader range of OM, including microbial metabolites and/or necromass. As soil aggregates form and undergo turnover, POM can be trapped, transforming into occluded POM (oPOM), thereby protecting OM and reducing its susceptibility to biological degradation. The interactions between reactive minerals and OM suggest that changes in reactive minerals and their dynamics play a pivotal role in regulating OC sequestration by facilitating the formation of MAOM and oPOM (Fig. 2). The reactive minerals can also bind organic compounds to form MAOM (chemical processes)76, acting as a nucleus for this sequestration. Moreover, reactive minerals, along with the newly formed MAOM with active surfaces, can physically bind free POM to form oPOM within soil aggregates (physical processes)77 (Fig. 2). Therefore, the composition and properties of reactive minerals should be given increased attention to gain a comprehensive understanding of the forms and status of OM stabilization in soil.
The RMS-based approach builds upon the most current insights and rationale regarding the various functions of reactive minerals in OM dynamics. It emphasizes coordination of both direct mineral-OM interactions and indirect interactions involving minerals, microorganisms, and plants, all within the context of relevant biological and abiological environmental factors (Fig. 3). The RMS strategy promotes the pivotal role of reactive minerals as an integrative and optimized solution for the long-term sequestration of OC in soil. The dynamics of reactive mineral transformation is closely linked to the soil’s weathering history and age, influenced by a range of abiotic conditions (e.g., climate, initial primary mineralogy) and biotic factors (e.g., microbial and plant growth). Therefore, it is necessary to consider the soil’s mineralogy in both young and mature soil systems when formulating strategies aimed at enhancing and mitigating the sequestration of OC in soils (Fig. 3).
Soil age and mineral dynamics
In young soils, like Cambisols, Cryosols and Podzols, early pedogenesis is in progress. These soils still contain abundant primary minerals that are actively undergoing weathering. Due to limited microbial and plant growth during the initial stages, these soils have relatively low levels of OM originating from biological sources (Fig. 3). Over the course of pedogenetic processes, the soil’s mineralogical composition, geochemical properties, and physical structure undergo dynamic changes, closely intertwined with the succession of plant and microbial communities. In contrast, mature soils, such as Acrisols, Lixisols, Ferralsols and Nitisols, have a relatively stable mineralogy. These soils have experienced extensive weathering processes and major pedogenic phases. Before erosive loss, mature soils are expected to contain an abundance of weathered primary minerals and secondary minerals. They exhibit relatively stable geochemical properties, physical structure, and host well-established plant and microbial communities. The RMS-based strategy is considered to be closely related to the primary mineral-dominated young soils and secondary mineral-dominated mature soils which drive the dynamics of soil OM.
Young soil
In young soils during early pedogenesis, an abundance of primary minerals still exists. These soils have only developed limited populations of biota, including microorganisms and lower plants, resulting in inherently low OC content. Therefore, enhancing the abundance and functionality of the biota is critical to initiating and accelerating pedogenetic processes and OC sequestration and storage. Biotas are powerful drivers in mineral weathering. Based on this premise, strategies aimed at restoring or rejuvenating the capacity of young soils to stabilize OM should take into account the interlinked processes illustrated in Fig. 3. These include: (1) Stimulating bio-weathering of primary minerals to generate secondary minerals with low crystallinity, thereby increasing the potential for mineral-OM reaction; (2) Promoting diverse plant and microbial successions to foster the generation of complex and long-lasting OM; and (3) Implementing eco-engineering approaches that facilitate the formation of aggregates containing reactive minerals where OM can be speciated and occluded or stored.
Enhancing mineral weathering
Enhancing mineral weathering in young soil or soils predominantly composed of primary minerals is considered as a fundamental requirement for increasing the soil’s capacity to sequester OC. In these substrates, the less stable primary minerals can be readily weathered, giving rise to secondary minerals which are essential constituents of the reactive mineral pool. These secondary minerals exhibit significantly increased surface area, leading to the formation of MAOM and the stabilization of OC. As mineral weathering and secondary mineral formation progress, these minerals can also drive chemical transformation of OM molecule profiles9. In a recent study conducted at the continental scale78, a strong link was made between primary mineral weathering rate and the geographic distribution of poorly crystalline minerals. This study revealed that soil OC was effectively stabilized in regions abundant in poorly crystalline minerals78, e.g., those mineral products resulting from the weathering of primary minerals. This work highlighted the importance of secondary minerals which are integral to the RMS in the sequestration and stabilization of soil OC. To stimulate mineral weathering and secondary mineral formation, and increase the reactivity of mineral substrates in the soil, microorganism proficient in mineral weathering26 and pioneering, as well as resilient plants with robust root system79, can be employed. These strategies lead to an increase in the quantity of OC adsorbed, transformed, and stabilized in MAOM. Ultimately, maintaining a certain rate of mineral weathering and transformation is critical to replenishing and maintaining the size and functionality of the RMS in the soil. This process can be enhanced by activating biota to sustain mineral weathering processes.
Diverse and persistent OM generation and inputs
In addition to mineral weathering, increasing inputs of a wide range of OM is critical to sustaining OC stabilization in young soils. This may be facilitated by promoting the colonization of native microbes and plants that are well-suited and adaptive to the physical and chemical conditions present in the young soil dominated with primary minerals. In harsh environments, biocrust, biofilm and mosses could establish a presence in young soils, contributing to an increase OM inputs80. Native pioneer plant species help to mobilize and recycle mineral nutrients inherent to the primary mineral. Their biomass litters enhance the development and growth of the microbial communities and biocrusts, which, in turn, leads to increased microbial-derived OM in soil. This includes microbial biomass, amino acids, sugars, chitin, lipids, and N-rich compounds such as proteins81. Notably, proteins are known to have a higher affinity for minerals compared to OM with lower N content22,40. It is worth highlighting that the interplay between root and microbial activities and the types of minerals present in the soil significantly influence OM generation, stabilization and loss35. Thus, it is imperative to systematically consider plant-microbe-mineral interactions when striving to enhance OC sequestration in young soils.
Stable aggregate formation
The processes of mineral weathering and the sustainable input of OM are fundamental to the formation of soil aggregates. These aggregates occlude POM and MAOM, enabling long-term storage of OC. The generation of secondary minerals is a critical factor in the durability of these cemented aggregate, which contributes to the stabilization of OC45. In young soils rich in poorly stable primary minerals, ecological engineering processes, such as the introduction of native plant species and the addition of plant litter as a source of OM, have been used to accelerate mineral weathering, foster organo-mineral associations, and promote aggregate formation33,45. These strategies have led to an improvement in the stabilization of OC through both occluded POM and MAOM82. Eco-engineering strategies can be devised to enhance RMS, and consequently, boost the sequestration and storage of OC in developing young soils.
Mature soil
Many types of mature or aged soils have experienced prolonged weathering processes, resulting in a high degree of crystallinity and relatively low reactivity. This diminished reactivity is often evident through metrics like the ratio of amorphous to crystalline Fe-minerals83. Regeneration of reactive minerals in mature soil represents an important strategy for restoring and rejuvenating their capacity to sequester OC. “Regeneration” in this context means to reactivate mineral transformation to enhance mineral reactivity which is required for mineral-microbial-organic interactions. This can be effectively achieved by adding reactive minerals, such as through eco-engineering practices, or through adding alluvium or colluvium, in addition to the involvement of soil biota and native plants (Fig. 3). Briefly, the strategies include: (1) Increasing mineral transformation and encouraging the neoformation of reactive minerals and/or the introduction of exogenous reactive mineral amendments to increase reaction sites for organo-mineral interactions through biological and abiological regulation. (2) Improving microbial assimilation of OM and increasing the biochemical persistence of OM by implementing efficient revegetation and microbiome management in soil. These actions should consider the existing mineral composition and properties. (3) Slowing down soil aggregate turnover and enhancing the occlusion and storage of POM and MAOM within aggregates using biological and abiological treatments. This strategy is particularly relevant for the restoration or rehabilitation of degraded soils, especially those where mineral reactivity and OC sequestration capacity have largely been diminished due to factors such as long-term intensive cropping, grazing, or mismanagement.
Activating mineral dynamics
Although minerals and MAOM in mature soil are relatively stable, they remain subject to dynamic change, such as dispersion, mineral transformation, and new organo-mineral association. These occur in response to seasonal wet and dry cycles as well as bioweathering processes driven by microbes and roots84,85,86. For instance, during wet seasons, shifts in soil redox conditions may influence the transformation of Fe and Mn minerals87, triggering alterations in the dynamics of OM chemistry and stabilization9. In mature soil that are enriched in Fe(III) minerals, heterotrophic bacteria such as Geobacter sp. stimulate the reduction of Fe(III), leading to the transformation of mineral phases and the initiation of new organo-mineral interactions88. Furthermore, according to the conceptual theory of organo-mineral association proposed by Kleber et al. (2007)22, increasing amounts of OM can be stabilized in the outer layer of the existing MAOM, forming MAOM clusters. These clusters consist of numerous MAOM components assembled through organo-organic and/or organo-mineral interactions89. This phenomenon enhances the mature soil’s capacity for sequestering OC90.
In the context of soil quality management within agriculture systems, replenishing reactive minerals into structurally degraded soils may be an effective strategy to enhance organo-mineral interactions and promoting the stabilization of OC91. In fact, multiple studies have shown that the addition of clay minerals (e.g., smectite, kaolinite, illite) and/or oxyhydroxides like ferrihydrite and allophane increased the long-term sequestration of OC in soil19. Therefore, it is proposed that soil amendments enriched with such minerals should be used as soil remedies to enhance the sequestration of OC in mature soil, particularly those that have been degraded or highly weathered. These mineral fertilizers not only provide essential nutrients (such as P, S, K, Ca, Mg) for the development of plants and microorganisms but they also provide more reactive binding sites for developing organo-mineral associations, thereby facilitating the long-term sequestration of OC. In summary, even mature soils, such as forest soils, still have the potential to substantially increase the sequestration of OC by encouraging the regeneration of reactive minerals and/or by introducing exogenous reactive mineral amendments (Fig. 3).
Improving OM biochemical persistence
Apart from RMS abundance in mature soil, the biochemical persistence of OM is important to long-term OC stabilization. This persistence can be achieved by increasing the presence of native plants and the microbiome in soil. For instance, mycorrhizal fungi working together with fine roots have the potential to increase overall input of OM and its chemical complexity92,93. Within agricultural soil systems, introducing natural conservation into cropping and pasture landscapes can improve bioturbation, OM diversity and enhance its stability94. The adoption of regenerative agriculture practices could also increase OC accumulation by bolstering the enrichment of both POM and MAOM95. Additionally, implementing crop rotations that include legumes and transitioning land use from cropping to pasture could increase OC sequestration73.
Among biota-derived OM sources, those derived from microorganisms are considered to be more persistent and prone to stabilization4. As a result, enriching microbial-derived OM formation is one of the important strategies to increase OC stabilization and storage in mature soil. For instance, well-managed and long-term grassland practices have been shown to increase the presence of microbial necromass in MAOM fractions when compared to annual cropping systems96. Likewise, multi-species intercropping has been found to increase microbial-derived OM accumulation in macroaggregates in contrast to mono-species cropping system97. The generation of microbial-derived OM is closely linked to the concept of carbon use efficiency (CUE) within the soil. Management of the soil microbiome can be facilitated by the presence of a reactive mineral matrix27,98, which can influence microbial communities and temporary microenvironmental variations like temperature, moisture, and oxygen. These factors, in turn, improve CUE, microbial OM production and the development of organo-microbial-mineral assemblages – factors that are crucial to long-term OC sequestration.
Enhancing OC storage in soil aggregates
In mature soils, it is important to protect and stabilize OC through enhancing soil aggregate stability and functional turnover77. For instance, biomass such as mycorrhizal fungi99,100, and/or mineral cements such as reactive minerals with high specific surface area45, can slow down the turnover of aggregates. This, in turn, enhances the protection of OC by occluding POM and MAOM with the aggregates.
Overall, the RMS-based strategy emphasizes the dynamics of reactive minerals and mineral-microbe/plant-OM interactions. These interactions play a pivotal role in influencing the biochemical persistence and stabilization of OM.
Advanced techniques for unraveling RMS-driven OM dynamics
Understanding the behavior of soil OM and its relationships with minerals presents a challenge when using conventional analytical approaches, primarily because of the intricate and heterogeneous nature of soil OM pool1. Previous research often relied on chemical extraction techniques to characterize humic substrates within soil, but these methods are limited in their ability to provide high-resolution and intrinsic insights into organo-mineral interactions101. Instead, advanced mass spectrometry (MS) and high resolution micro-spectroscopic analysis methods are required to examine the interfaces between microbes-minerals-OM. Such techniques will unravel the relationships among OM transformation and stabilization and mineral reactivity in soil OC storage and cycling101,102.
Ultrahigh-resolution analytical techniques, such as Electrospray ionization ultrahigh-resolution Fourier transform cyclotron resonance mass spectrometry (ESI-FT-ICR-MS)103 and Orbitrap-MS104, are highly effective in deciphering mineral-mediated OM reactions by capturing the products of newly formed organic molecules at the molecular level. These methodologies provide valuable insights into OM chemodiversity and composition103. High resolution MS can also reveal the fractionation of organic molecules resulting from the preferential adsorption of specific molecules by minerals105. Using FT-ICR-MS, we have recently uncovered thousands of organic molecules and observed molecular shifts associated with the weathering of Fe-bearing minerals in ore tailings59. However, it is important to note that high-resolution MS techniques are not suitable for characterizing the forms of OM interfacing with minerals at the micro-and nanoscale.
To complement the FT-ICR-MS method, advanced techniques such as synchrotron-based scanning transmission X-ray microscopy (STXM) coupled to near edge X-ray absorption fine structure spectroscopy (NEXAFS) and nanoscale secondary ion mass spectrometry (NanoSIMS) can be used to resolve OC forms and their relationship with minerals at micro- and nano-scale106. A range of imaging techniques, including high resolution micro-computed tomography (CT)107, synchrotron-based X-ray fluorescence microscopy (XFM)45,108, and micro-Fourier transform infrared spectrometry (FTIR) mapping109, can be utilized for in situ characterization of soil architecture40,110. These methods enable the study of organic and mineral distribution in relation to the pore network, providing valuable insights into soil structure and functions. Information obtained through such in situ high resolution micro-spectroscopic analyses offers direct evidence of the spatial distribution and chemical properties of reactive minerals and their association with OM. This aids in the characterization of soil architecture and fine-scale structure. Furthermore, such spatial imaging data, collected over a period, can track the dynamic movement and transformation of minerals and OM in response to various treatments in the soil. This is especially critical for gaining an in-depth understanding of OM dynamics and soil structure development driven by reactive minerals. However, it is recognized that micro-spectroscopic methods can only capture signals of OC distribution and/or overall OC forms. They do not provide information about the quantity and molecular composition of OM profile. Therefore, a combination of a suite of high-resolution MS and micro-spectroscopic methodologies, together with macroanalyses (bulk, physical, chemical fractionation)111, is in our opinion the best way forward for unraveling the processes and mechanisms of RMS-driven OM dynamics and stabilization in soil.
Concluding remarks
The RMS model advocates the previously overlooked yet crucial role of reactive minerals in governing the dynamics, longevity, and stabilization of OM in soil. This novel perspective emphasizes the significance of RMS in preserving and revitalizing soil OM storage and biogeochemical functionality through a combination of biological, physical, and chemical processes. The RMS-based strategy takes into consideration the interplay between reactive minerals and OM derived from plants and microorganisms. This holistic approach provides a new pathway for further research on enhancing the biochemical persistence and stabilization of OM, distinguishing itself from other approaches that have primarily emphasized either MAOM or POM112.
In the RMS-based strategy, we advocate for a coordinated approach that considers various factors related to management and treatment, including mineral availability, OM inputs and transformations, and the stability of the soil aggregates. Moreover, RMS highlights the critical importance of soil mineralogy in estimating soil OC sequestration and storage as a means of mitigating climate change – an area that has largely been overlooked in conventional C modeling73. Further studies by soil OC researchers will be essential to enrich the much-needed data and validate the RMS model. A world-wide initiative involving sampling and investigation can compile a comprehensive database on OM dynamics across diverse soil types, particularly in relation to the abundance and composition of reactive minerals.
Given the intricate spatial and functional complexity of soil mineral matrix and its ever-changing nature due to mineral weathering, it is imperative to employ a suite of cutting-edge methodologies, including high-resolution mass spectrometry and various micro-spectroscopic techniques, to unravel the dynamics and stabilization mechanisms driven by RMS under a variety of environmental conditions. The insights gained from the RMS model form the fundamental basis for advancing soil health, promoting sustainable food and fiber production, and reinforcing the resilience of terrestrial ecosystems.
Data availability
All the information mentioned in this manuscript is available in the main text.
References
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Wiesmeier, M. et al. Soil organic carbon storage as a key function of soils - A review of drivers and indicators at various scales. Geoderma 333, 149–162 (2019).
Schmidt, M. W. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nature microbiology 2, 1–6 (2017).
Hemingway, J. D. et al. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570, 228–231 (2019).
Coward, E. K., Ohno, T. & Plante, A. F. Adsorption and molecular fractionation of dissolved organic matter on iron-bearing mineral matrices of varying crystallinity. Environ. Sci. Technol. 52, 1036–1044 (2018).
Gu, B., Schmitt, J., Chen, Z., Liang, L. & McCarthy, J. F. Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ. Sci. Technol. 28, 38–46 (1994).
Xiao, K.-Q. et al. Introducing the soil mineral carbon pump. Nat. Rev. Earth Environ. 4, 135–136 (2023).
Kleber, M. et al. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2, 402–421 (2021).
Huang, P.-M., Wang, M.-K. & Chiu, C.-Y. Soil mineral–organic matter–microbe interactions: Impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 49, 609–635 (2005).
Dong, H. et al. A critical review of mineral-microbe interaction and co-evolution: mechanisms and applications. Natl. Sci. Rev. 9, nwac128 (2022).
Uroz, S., Kelly, L. C., Turpault, M. P., Lepleux, C. & Frey-Klett, P. The mineralosphere concept: mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol. 23, 751–762 (2015).
Six, J., Bossuyt, H., Degryze, S. & Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 79, 7–31 (2004).
Totsche, K. U. et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 181, 104–136 (2018).
Cemič, L. Thermodynamics in mineral sciences. (Springer-Verlag, 2005).
Blanc, P. et al. Thermodynamics for clay minerals: Calculation tools and application to the case of illite/smectite interstratified minerals. Appl. Geochem. 130, 104986 (2021).
Vieillard, P. A new method for the prediction of Gibbs free energies of formation of hydrated clay minerals based on the electronegativity scale. Clays Clay Miner 48, 459–473 (2000).
Beckingham, L. E. et al. Evaluation of mineral reactive surface area estimates for prediction of reactivity of a multi-mineral sediment. Geochim. Cosmochim. Acta 188, 310–329 (2016).
Singh, M. et al. in Advances in Agronomy Vol. 148 Advances in Agronomy 33-84 (2018).
Kögel-Knabner, I. & Amelung, W. Soil organic matter in major pedogenic soil groups. Geoderma 384, 114785 (2021).
Mahmoudi, N., Steen, A. D., Halverson, G. P. & Konhauser, K. O. Biogeochemistry of Earth before exoenzymes. Nat. Geosci. 16, 845–850 (2023).
Kleber, M., Sollins, P. & Sutton, R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85, 9–24 (2007).
Schoonen, M. A. et al. Mineral-induced formation of reactive oxygen species. Rev. Mineral. Geochem. 64, 179 (2006).
Hartmann, M. & Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 4, 4–18 (2022).
Kravchenko, A. N. & Guber, A. K. Soil pores and their contributions to soil carbon processes. Geoderma 287, 31–39 (2017).
Uroz, S., Picard, L. & Turpault, M. P. Recent progress in understanding the ecology and molecular genetics of soil mineral weathering bacteria. Trends Microbiol. 30, 882–897 (2022).
Kallenbach, C. M., Frey, S. D. & Grandy, A. S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 7, 13630 (2016).
Bryce, C. et al. Microbial anaerobic Fe (II) oxidation–ecology, mechanisms and environmental implications. Environ. Microbiol. 20, 3462–3483 (2018).
Xi, W. et al. Reduced Iron-Containing Clay Minerals as Antibacterial Agents. (2017).
Vatansever, F. et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 37, 955–989 (2013).
Dong, H. et al. Coupled iron cycling and organic matter transformation across redox interfaces. Nat. Rev. Earth. Environ. 4, 659–673 (2023).
Weber, K. A., Achenbach, L. A. & Coates, J. D. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4, 752–764 (2006).
Wu, S. et al. Organic matter amendment and plant colonization drive mineral weathering, organic carbon sequestration, and water-stable aggregation in magnetite fe ore tailings. Environ. Sci. Technol. 53, 13720–13731 (2019).
Fang, Q. et al. Mineral weathering is linked to microbial priming in the critical zone. Nat. Commun. 14, 345 (2023).
Liang, G., Stark, J. & Waring, B. G. Mineral reactivity determines root effects on soil organic carbon. Nat. Commun. 14, 4962 (2023).
Neurath, R. A. et al. Root carbon interaction with soil minerals is dynamic, leaving a legacy of microbially derived residues. Environ. Sci. Technol. 55, 13345–13355 (2021).
Kleber, M. et al. Mineral–organic associations: formation, properties, and relevance in soil environments. Adv. Agron. 130, 1–140 (2015).
Steffens, M. et al. Identification of distinct functional microstructural domains controlling c storage in soil. Environ. Sci. Technol. 51, 12182–12189 (2017).
Jeewani, P. H. et al. Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients. Soil Biol. Biochem. 160, 108312 (2021).
Wu, S. et al. Nitrogen-rich organic matter formation and stabilization in iron ore tailings: a submicrometer investigation. Environ. Sci. Technol. 57, 12325–12338 (2023).
Xu, Y. et al. Formation efficiency of soil organic matter from plant litter is governed by clay mineral type more than plant litter quality. Geoderma 412, 115727 (2022).
Tamrat, W. Z. et al. Soil organo-mineral associations formed by co-precipitation of Fe, Si and Al in presence of organic ligands. Geochim. Cosmochim. Acta 260, 15–28 (2019).
ThomasArrigo, L. K., Vontobel, S., Notini, L. & Nydegger, T. Coprecipitation with ferrihydrite inhibits mineralization of glucuronic acid in anoxic soils. Environ. Sci. Technol. 57, 9204–9213 (2023).
Fritzsche, A. et al. Structure and composition of Fe–OM co-precipitates that form in soil-derived solutions. Geochim. Cosmochim. Acta 169, 167–183 (2015).
Wu, S. et al. Ecological engineering of iron ore tailings into useable soils for sustainable rehabilitation. iScience 26, 107102 (2023).
Chorover, J. & Amistadi, M. K. Reaction of forest floor organic matter at goethite, birnessite and smectite surfaces. Geochim. Cosmochim. Acta 65, 95–109 (2001).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature nanotechnology 2, 577–583 (2007).
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Moore, O. W. et al. Long-term organic carbon preservation enhanced by iron and manganese. Nature 621, 312–317 (2023).
Naughton, H. R. et al. Reactive iron, not fungal community, drives organic carbon oxidation potential in floodplain soils. Soil Biol. Biochem. 178, 108962 (2023).
Trusiak, A., Treibergs, L. A., Kling, G. W. & Cory, R. M. The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim. Cosmochim. Acta 224, 80–95 (2018).
Yu, G.-H. & Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci. Rev. 214, 103525 (2021).
Han, R. et al. Multiple effects of humic components on microbially mediated iron redox processes and production of hydroxyl radicals. Environ. Sci. Technol. 56, 16419–16427 (2022).
Wang, T., Tian, Z., Bengtson, P., Tunlid, A. & Persson, P. Mineral surface‐reactive metabolites secreted during fungal decomposition contribute to the formation of soil organic matter. Environ. Microbiol. 19, 5117–5129 (2017).
Tong, M. et al. Production of abundant hydroxyl radicals from oxygenation of subsurface sediments. Environ. Sci. Technol. 50, 214–221 (2016).
Xu, J., Sahai, N., Eggleston, C. M. & Schoonen, M. A. Reactive oxygen species at the oxide/water interface: Formation mechanisms and implications for prebiotic chemistry and the origin of life. Earth Planet. Sci. Lett. 363, 156–167 (2013).
Lehmann, J. et al. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 1, 238–242 (2008).
Qian, A. et al. Mechanistic Insight into Electron Transfer from Fe(II)-Bearing Clay Minerals to Fe (Hydr)oxides. Environ. Sci. Technol. 57, 8015–8025 (2023).
Wu, S. et al. Chemodiversity of dissolved organic matter and its molecular changes driven by rhizosphere activities in fe ore tailings undergoing eco-engineered pedogenesis. Environ. Sci. Technol. 55, 13045–13060 (2021).
Chi, J., Fan, Y., Wang, L., Putnis, C. V. & Zhang, W. Retention of soil organic matter by occlusion within soil minerals. Rev. Environ. Sci. Bio/Technol. 21, 727–746 (2022).
Konhauser, K. O. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 43, 91–121 (1998).
Konhauser, K. O. & Urrutia, M. M. Bacterial clay authigenesis: a common biogeochemical process. Chem. Geol. 161, 399–413 (1999).
Vogel, H. J. et al. A holistic perspective on soil architecture is needed as a key to soil functions. Eur. J. Soil Sci. 73, e13152 (2021).
Assouline, S. & Or, D. Conceptual and parametric representation of soil hydraulic properties: A review. Vadose Zone Journal 12 (2013).
Wang, Y., Bradford, S. A. & Šimůnek, J. Transport and fate of microorganisms in soils with preferential flow under different solution chemistry conditions. Water Resour. Res. 49, 2424–2436 (2013).
Roth, V.-N. et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat. Geosci. 12, 755–761 (2019).
Rillig, M. C., Muller, L. A. & Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 11, 1943–1948 (2017).
ThomasArrigo, L. K., Byrne, J. M., Kappler, A. & Kretzschmar, R. Impact of organic matter on iron (II)-catalyzed mineral transformations in ferrihydrite–organic matter coprecipitates. Environ. Sci. Technol. 52, 12316–12326 (2018).
Hoffland, E., Kuyper, T. W., Comans, R. N. & Creamer, R. E. Eco-functionality of organic matter in soils. Plant Soil 455, 1–22 (2020).
Wu, S. et al. Deficiencies of secondary Fe (oxy)hydroxides associated with phyllosilicates and organic carbon limit the formation of water-stable aggregates in Fe-ore tailings. Chem. Geol. 523, 73–87 (2019).
Lehmann, J., Bossio, D. A., Kogel-Knabner, I. & Rillig, M. C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 1, 544–553 (2020).
Moebius-Clune, B. et al. Comprehensive assessment of soil health–the Cornell framework manual, edition 3.0. Cornell University, Geneva. preparation, http://soilhealth. cals. cornell. edu (2015).
Stockmann, U. et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 164, 80–99 (2013).
Mikutta, R., Kleber, M., Torn, M. S. & Jahn, R. Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77, 25–56 (2006).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261–273 (2020).
Kögel-Knabner, I. et al. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 171, 61–82 (2008).
Six, J., Paustian, K., Elliott, E. T. & Combrink, C. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 64, 681–689 (2000).
Slessarev, E. W., Chadwick, O. A., Sokol, N. W., Nuccio, E. E. & Pett-Ridge, J. Rock weathering controls the potential for soil carbon storage at a continental scale. Biogeochemistry 157, 1–13 (2021).
Wu, S. et al. Rhizosphere drives biotite-like mineral weathering and secondary fe–si mineral formation in fe ore tailings. ACS Earth Space Chem. 5, 618–631 (2021).
Eldridge, D. J. et al. The global contribution of soil mosses to ecosystem services. Nat. Geosci. 16, 430–438 (2023).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105 (2017).
Li, Z. et al. Arbuscular mycorrhizal symbiosis enhances water stable aggregate formation and organic matter stabilization in Fe ore tailings. Geoderma 406, 115528 (2022).
Duiker, S. W., Rhoton, F. E., Torrent, J., Smeck, N. E. & Lal, R. Iron (hydr) oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 67, 606–611 (2003).
Huang, L. et al. Molecular determination of organic adsorption sites on smectite during Fe Redox processes using ToF-SIMS analysis. Environ. Sci. Technol. 55, 7123–7134 (2021).
Zeng, Q. et al. Mutual interactions between reduced Fe-bearing clay minerals and humic acids under dark, oxygenated conditions: hydroxyl radical generation and humic acid transformation. Environ. Sci. Technol. 54, 15013–15023 (2020).
Chen, C., Hall, S. J., Coward, E. & Thompson, A. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 11, 2255 (2020).
Mejia, J., Roden, E. E. & Ginder-Vogel, M. Influence of oxygen and nitrate on Fe (hydr) oxide mineral transformation and soil microbial communities during redox cycling. Environ. Sci. Technol. 50, 3580–3588 (2016).
Zhang, X. et al. Exogenous electroactive microbes regulate soil geochemical properties and microbial communities by enhancing the reduction and transformation of Fe(III) minerals. Environ. Sci. Technol. 57, 7743–7752 (2023).
Possinger, A. R. et al. Organo-organic and organo-mineral interfaces in soil at the nanometer scale. Nat. Commun. 11, 6103 (2020).
Begill, N., Don, A. & Poeplau, C. No detectable upper limit of mineral-associated organic carbon in temperate agricultural soils. Glob. Chang. Biol. 29, 4662–4669 (2023).
Zou, Z. et al. Decadal application of mineral fertilizers alters the molecular composition and origins of organic matter in particulate and mineral-associated fractions. Soil Biol. Biochem. 182, 109042 (2023).
Lang, A. K., Jevon, F. V., Vietorisz, C. R., Ayres, M. P. & Hatala Matthes, J. Fine roots and mycorrhizal fungi accelerate leaf litter decomposition in a northern hardwood forest regardless of dominant tree mycorrhizal associations. New Phytol. 230, 316–326 (2021).
Miller, R. & Jastrow, J. Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol. Biochem. 22, 579–584 (1990).
Wang, L., Pedersen, P. B. M. & Svenning, J.-C. Rewilding abandoned farmland has greater sustainability benefits than afforestation. npj Biodiversity 2, 5 (2023).
Prairie, A. M., King, A. E. & Cotrufo, M. F. Restoring particulate and mineral-associated organic carbon through regenerative agriculture. Proc. Nat. Acad. Sci. 120, e2217481120 (2023).
Rui, Y. et al. Persistent soil carbon enhanced in Mollisols by well-managed grasslands but not annual grain or dairy forage cropping systems. Proc. Natl. Acad. Sci. USA. 119, e2118931119 (2022).
Zhao, X. et al. Intercropping increases soil macroaggregate carbon through root traits induced microbial necromass accumulation. Soil Biol. Biochem. 185, 109146 (2023).
Fierer, N. & Walsh, C. M. Can we manipulate the soil microbiome to promote carbon sequestration in croplands? PLoS Biol. 21, e3002207 (2023).
Rillig, M. C. & Mummey, D. L. Mycorrhizas and soil structure. New Phytol. 171, 41–53 (2006).
Wu, S. et al. Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi - an updated conceptual framework. New Phytol. https://doi.org/10.1111/nph.19178 (2023).
Kögel-Knabner, I. & Rumpel, C. in Advances in Agronomy Vol. 149 Advances in Agronomy 1-48 (2018).
Lv, J., Huang, Z., Luo, L., Zhang, S. & Wang, Y. Advances in molecular and microscale characterization of soil organic matter: current limitations and future prospects. Environ. Sci. Technol. 56, 12793–12810 (2022).
Bahureksa, W. et al. Soil organic matter characterization by fourier transform ion cyclotron resonance mass spectrometry (FTICR MS): a critical review of sample preparation, analysis, and data interpretation. Environ. Sci. Technol. 55, 9637–9656 (2021).
Perry, R. H., Cooks, R. G. & Noll, R. J. Orbitrap mass spectrometry: instrumentation, ion motion and applications. Mass Spectrom. Rev. 27, 661–699 (2008).
Lv, J. et al. Molecular-scale investigation with ESI-FT-ICR-MS on fractionation of dissolved organic matter induced by adsorption on iron oxyhydroxides. Environ. Sci. Technol. 50, 2328–2336 (2016).
Behrens, S., Kappler, A. & Obst, M. Linking environmental processes to the in situ functioning of microorganisms by high-resolution secondary ion mass spectrometry (NanoSIMS) and scanning transmission X-ray microscopy (STXM). Environ. Microbiol. 14, 2851–2869 (2012).
Ma, R. et al. Evaluation of soil aggregate microstructure and stability under wetting and drying cycles in two Ultisols using synchrotron-based X-ray micro-computed tomography. Soil Tillage Res. 149, 1–11 (2015).
Morris, E. K. et al. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 13, 1639–1646 (2019).
Kaminskyj, S., Jilkine, K., Szeghalmi, A. & Gough, K. High spatial resolution analysis of fungal cell biochemistry–bridging the analytical gap using synchrotron FTIR spectromicroscopy. FEMS Microbiol. Lett. 284, 1–8 (2008).
Remusat, L. et al. NanoSIMS study of organic matter associated with soil aggregates: advantages, limitations, and combination with STXM. Environ. Sci. Technol. 46, 3943–3949 (2012).
von Lützow, M. et al. SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 39, 2183–2207 (2007).
Angst, G. et al. Unlocking complex soil systems as carbon sinks: multi-pool management as the key. Nat. Commun. 14, 2967 (2023).
Acknowledgements
This work is financially supported by Australian Research Council Linkage Projects (LP160100598 and LP190100975). Thanks to Prof Yong-Guan Zhu and Dr Ke-Qing Xiao for their valuable suggestions which help improve the manuscript.
Author information
Authors and Affiliations
Contributions
S.W.: Conceptualization, Methodology, Discussion, Writing-original draft, review & editing. K.O.K.: Discussion, Writing-review & editing. B.C.: Discussion, Writing- review & editing. L.H.: Conceptualization, Discussion, Funding acquisition, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, S., Konhauser, K.O., Chen, B. et al. “Reactive Mineral Sink” drives soil organic matter dynamics and stabilization. npj Mater. Sustain. 1, 3 (2023). https://doi.org/10.1038/s44296-023-00003-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44296-023-00003-7