Abstract
Antimicrobial peptides (AMPs) are key components of innate immunity across all domains of life. Natural and synthetic AMPs are receiving renewed attention in efforts to combat the antimicrobial resistance (AMR) crisis and the loss of antibiotic efficacy. The gram-negative pathogen Pseudomonas aeruginosa is one of the most concerning infecting bacteria in AMR, particularly in people with cystic fibrosis (CF) where respiratory infections are difficult to eradicate and associated with increased morbidity and mortality. Cationic AMPs exploit the negatively charged lipopolysaccharides (LPS) on P. aeruginosa to bind and disrupt bacterial membrane(s), causing lethal damage. P. aeruginosa modifies its LPS to evade AMP killing. Free-LPS is also a component of CF sputum and feeds pro-inflammatory cycles. Glatiramer acetate (GA) is a random peptide co-polymer—of glycine, lysine, alanine, tyrosine—used as a drug in treatment of multiple sclerosis (MS); we have previously shown GA to be an AMP which synergises with tobramycin against CF P. aeruginosa, functioning via bacterial membrane disruption. Here, we demonstrate GA’s direct binding and sequestration/neutralisation of P. aeruginosa LPS, in keeping with GA’s ability to disrupt the outer membrane. At CF-relevant LPS concentrations, however, membrane disruption by GA was not strongly inhibited. Furthermore, exposure to GA did not result in increased Lipid A modification of LPS or in increased gene expression of systems involved in AMP sensing and LPS modification. Therefore, despite the electrostatic targeting of LPS by GA as part of its activity, P. aeruginosa does not demonstrate LPS modification in its defence.
Similar content being viewed by others
Introduction
The efficacy of antibiotics in treating bacterial infections is decreasing due to antimicrobial resistance (AMR) and the costs continue to mount globally in morbidity and mortality1,2,3,4,5. New tools and strategies are required to deal with increasing AMR, particularly in the absence of development and approval of new antibiotics6,7,8,9. Pseudomonas aeruginosa is of particular concern in AMR due to its innate, acquired and adaptive resistance mechanisms. It is considered by the World Health Organisation (WHO) to be of Critical priority for development of new antimicrobials10. P. aeruginosa is a ubiquitous environmental gram-negative bacterium which infects opportunistically across a variety of bodily sites particularly when host defences have been compromised11.
P. aeruginosa is especially associated with infections in the lungs of people with cystic fibrosis (CF) where it results in both acute and chronic infections with periods of exacerbation and results in worse long-term outcomes12,13,14,15,16. Antibiotic treatments of infections in CF pose their own, unique set of challenges, on top of those of AMR, including the physical barrier of the characteristic dehydrated CF airway surface liquid, an acidified environment and the recalcitrant lifestyle of the bacterial biofilm17,18,19.
In response to the demands of the AMR crisis, attention has increasingly turned to antimicrobial peptides (AMPs); short, usually cationic and amphipathic peptides which occur naturally across the domains of life as a central part of innate immunity20,21. AMPs mostly function as bacterial membrane disruptors, weakening or breaching the bacterial cell envelope (CE) and causing membrane collapse and cell death. The ‘antibiotic-of-last-resort’ colistin (CST) is an AMP which has increasingly come to prominence in response to the loss of efficacy of other antibiotic classes22. Many AMPs (both natural and synthetic) are currently in development or in trials as direct acting agents or as antibiotic adjuvants8,23. While few AMPs have made it to the clinic to-date, predominantly for issues of host cell cytotoxicity at antibacterial concentrations, they have potential advantages over other antimicrobial classes in terms of resistance generation which has generally been shown to occur less frequently with AMPs24,25,26.
Breaching the membrane of gram-negative bacteria is a major challenge for antibiotic treatments; with two membranes and the added protection of lipopolysaccharides (LPS) embedded in the outer membrane (OM), accessing the cell cytoplasm is extremely difficult for antimicrobials27. Cationic AMPs attack bacterial cells through electrostatic interactions with the CE; the positively charged AMP attaches to the negatively charged membrane, often via the outermost aspect of the cell, the LPS in the case of P. aeruginosa21,28,29. This electrostatic attachment of AMPs facilitates hydrophobic insertion into the lipid bilayer and membrane disruption. As a defence, P. aeruginosa strains can modify their LPS structures by addition of positive charges, neutralising the charge of the cell and reducing AMP binding affinity30. This can take the form of exploiting cations in their environment (preferentially divalent cations Mg2+ and Ca2+) or by genetically encoded mechanisms31,32. The best studied of these modifications is the addition of 4-amino-4-deoxy-L-arabinose (L-Ara4N) to the Lipid A portion of LPS which is carried out by the Arn operon (ArnBCADTEF) and mediated by a series of two component systems (TCSs) of P. aeruginosa (PhoPQ, PmrAB, CprSR, ParSR and ColSR)33. Mutations in these TCSs lead to AMP resistance with true CST resistance resulting from changes which deactivate TCS sensor gene(s) leading to constitutive Arn operon expression and LPS modification32,34,35,36,37,38. As well as L-Ara4N, other encoded Lipid A modification types include additions of phosphate, C10:3OH species, palmitate and combinations of these with L-Ara4N- and palmitate-modified LPS being associated with increased airway disease severity32,39.
Free-LPS (shed by bacteria and from dead bacteria) is a significant component of the sputum of people with CF, as is extracellular DNA (eDNA), with both having been shown to have detrimental effects on the host40,41. LPS is highly pro-inflammatory and its presence in the CF lung contributes to cycles of inflammation seen in that environment and eDNA is a vital component of bacterial biofilms, promoting biofilm development and bacterial recalcitrance. Both LPS and eDNA feed into cycles of infection and inflammation in CF42,43,44,45. Presence of both may also have consequences for AMP activity in the CF lung, as they are negatively charged and capable of binding and sequestering cationic AMPs as well as competing for divalent cations46,47. Conversely, the affinity of AMPs for LPS has been proposed as being beneficial, leading to sequestration and neutralisation of free-LPS thereby limiting its pro-inflammatory capacity48,49.
We have previously shown in vitro that the multiple sclerosis (MS) drug glatiramer acetate (GA) also functions as an AMP and synergises with the aminoglycoside antibiotic, tobramycin, against P. aeruginosa from people with CF50,51. That work also showed GA is a bacterial membrane disruptor, in common with many AMPs21. Produced by the random polymerisation of the four N-carboxy-α-amino acid anhydrides of L-glutamate, L-lysine, L-alanine, and L-tyrosine, in MS GA functions as an immunomodulator52,53,54,55. In MS treatment, GA is administered subcutaneously54,56. Its mechanisms of action are incompletely understood in that condition but it is understood to be anti-inflammatory; GA treated macrophages and monocytes produce increased amounts of anti-inflammatory cytokines, GA treated dendritic cells promote the switch from pro-inflammatory T-helper 1 (Th1) cells towards anti-inflammatory Th2 cells and GA has also been shown to restore the functioning of immune cells which are known to be deficient in autoimmune conditions, such as MS57,58,59,60.
Having previously demonstrated GA’s ability to permeabilise P. aeruginosa cells and breach the CE while sensitising them to antibiotic treatment, here, we examine whether GA interacts with P. aeruginosa LPS via electrostatic interaction as its point of contact with the bacterial cell in common with other cationic AMPs. With the known interplay at a variety of levels between AMPs, LPS, divalent cations and eDNA and the presence and importance of each in the lungs of people with CF, it is necessary to understand their implications for GA activity in the CF airway. We also tested whether exposure to GA resulted in P. aeruginosa mounting a defensive response in the manner seen for other AMPs with modification of LPS and Lipid A. With a proposed benefit of AMPs being the decreased likelihood with which they generate resistance, it is important to know if GA is stimulatory to the TCSs commonly associated with AMP protection and LPS modification and potentially selective for resistance.
Results
Bilateral sequestration of GA and P. aeruginosa LPS
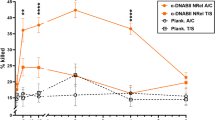
We first tested whether GA interacted directly with P. aeruginosa LPS as a potential mechanism of action of the drug. A known active concentration of GA (50 mg/L) was incubated with concentrations of LPS at supraphysiological level (0.1 mg/mL) and two concentrations relevant to the CF lung environment (0.02 and 0.01 mg/mL) at 37 °C for 30 min39,50,51,61,62. We determined the availability of free LPS with an Endotoxin detection kit reasoning that if GA targets LPS via electrostatic interactions the resulting GA-LPS complex would reduce the availability of LPS for quantification, as has been shown for other AMPs48,63,64,65. GA significantly neutralised LPS at concentrations of 0.02 (56.9 ± 4.7%) and 0.01 mg/mL (50.7 ± 12.8%) (p < 0.05) indicating direct binding of GA to LPS (Fig. 1a. Supplementary Fig. 1).
a Neutralisation of P. aeruginosa LPS was calculated as the percentage reduction of the quantifiable LPS in the presence of GA at each LPS concentration. Incubation of 0.02 (p = 0.0186) and 0.01 mg/mL (p = 0.0499) LPS with GA significantly increased neutralisation of LPS, compared to no GA (Kruskal-Wallis test with Dunn’s multiple comparison). (Median with 95%CI. n = 3). b The ability of 50 mg/L GA to disrupt the Outer Membrane of P. aeruginosa type strains was significantly reduced by 30 min pre-incubation with all P. aeruginosa LPS concentrations tested (each p < 0.0001). c The ability of 50 mg/L GA to depolarise the Cytoplasmic Membrane of P. aeruginosa type strains was significantly reduced by incubation with P. aeruginosa LPS concentration of 0.1 mg/mL (p < 0.0001) but not 0.02 mg/mL or 0.01 mg/mL. d The ability of 50 mg/L GA to permeabilise the Cell Envelope of P. aeruginosa type strains was significantly reduced by incubation with all P. aeruginosa LPS concentrations tested – 0.1 (p = 0.0002), 0.02 (p = 0.0024) and 0.01 mg/mL (p = 0.0031). b–d Log transformed data tested using Welch ANOVA with Dunnett’s T3 multiple comparison. Medians with 95% CIs of biological replicates (n = 9) of P. aeruginosa PAO1 (◯), PA14 (☐) or PAK (△).
The converse, sequestration of GA by LPS, was tested for using GA’s known membrane disruption properties as a proxy, due to the difficulties in accurately assaying for a highly heterogeneous peptide such as GA which can take on >1030 different peptide chains51. We assessed the impact on the OM, cytoplasmic membrane (CM) and on the overall CE permeability using P. aeruginosa LPS at the same concentrations: supraphysiological (0.1 mg/mL) and CF-lung-relevant (0.02 and 0.01 mg/mL). Disruption of the OM and permeabilisation of the CE of P. aeruginosa type strains by GA was significantly reduced after GA had been pre-incubated (37 °C for 30 min) with LPS (p < 0.01) (Fig. 1b, d. Supplementary Figs. 2a, 4a). The highest concentration of LPS tested also significantly reduced the ability of GA to depolarise the bacterial CMs (p < 0.0001) (Fig. 1c. Supplementary Fig. 3a). These results indicate that available concentrations of both LPS and GA are reduced by presence of other and that sequestration is bilateral.
Membrane perturbing activity of glatiramer acetate in the presence of CF-physiological P. aeruginosa LPS and divalent cations concentrations
With the evidence of direct interactions between GA and LPS, and the impact that pre-incubation had on GA’s action on bacterial cell membranes, we next tested the ability of GA to function if P. aeruginosa LPS was present in the background, as it will be in the CF lung environment. To do this, the previously reported membrane perturbation properties of GA were examined with LPS in the assay buffer, to test the effect of LPS presence on the known rapid action of GA on bacterial membranes. P. aeruginosa LPS significantly reduced the OM disruption of strains PAO1, PA14 and PAK at each concentration tested and the highest concentration also reduced CM depolarisation (p ≤ 0.05) (Fig. 2a, b. Supplementary Figs. 2b, 3b). No effect was seen on the ability of GA to permeabilise the CEs at the LPS concentrations tested (Fig. 2c. Supplementary Fig. 4b).
a Disruption of the OM of P. aeruginosa by GA was significantly reduced by each concentration of LPS tested (each p < 0.0001) while Mg2+ at 1 (p = 0.0051) and 0.5 mM (p = 0.0081) both also reduced GA activity. b Depolarisation of the CMs of P. aeruginosa by GA was only significantly reduced by LPS at the supraphysiological concentration of 0.1 mg/mL (p = 0.001) and was unaltered by Mg2+ presence. c Permeabilisation of the CE of P. aeruginosa by GA was not seen for any LPS concentration tested but was significantly reduced by Mg2+ (each p < 0.0001). Log transformed data tested using Welch ANOVA with Dunnett’s T3 multiple comparison. Medians with 95% CIs of biological replicates (n = 9) of P. aeruginosa PAO1 (◯), PA14 (☐) or PAK (△).
The protective properties of Mg2+ against GA activity were also tested at physiological concentrations at mid—(0.5 mM) and upper-levels (1 mM)66,67. Both concentrations of Mg2+ were protective against GA OM disruption and CE permeabilisation, with significant decreases in GA activity seen (p < 0.05) (Fig. 2a, c. Supplementary Figs. 2c, 4c), but this was not the case for depolarisation of the CM where no change was seen (Fig. 2b. Supplementary Fig. 3c). These results further support the evidence for cellular LPS as a target for GA binding by indicating competitive displacement between the two cations.
GA binds directly to DNA
We next wanted to test interactions of GA and DNA, due to their opposing charges, previous indications of GA-DNA aggregation and the high concentrations of eDNA in CF sputum playing a role in chronic biofilm formation50,68. With both supraphysiological (10 mg/mL) and physiological (1 mg/mL) concentrations of DNA, GA reduced detectable DNA using PI fluorescence in a dose-responsive fashion. Thus, as GA concentrations increased, detectable DNA decreased, reaching statistical significance by 25 mg/L (p < 0.05) (Fig. 3a. Supplementary Fig. 5). This indicates binding and sequestration of DNA by GA.
a Neutralisation of DNA was calculated as the percentage reduction of the detectable DNA in the presence of GA at each DNA concentration. All GA concentrations >25 mg/L significantly increased DNA neutralisation. At 1 mg/mL DNA, GA at 25 (p = 0.0111), 50 (p = 0.0068) and 100 mg/mL (p < 0.0001) while at 10 mg/mL DNA, GA at 25 (p = 0.0223), 50 (p = 0.0027) and 100 mg/mL (p < 0.0001) showed neutralisation (Kruskal-Wallis test with Dunn’s multiple comparison). (Medians with 95%CI. n = 5). The CF-relevant concentration of 1 mg/mL DNA did not alter the ability of 50 mg/L GA to disrupt the OM (b), depolarise the CM (c) or permeabilise the CE (d) of P. aeruginosa PAO1 (◯), PA14 (☐) or PAK (△). Welch’s t test of Log transformed data. Medians with 95%CIs of biological replicates (n = 9).
Similarly, to the experiments with LPS, we questioned whether such sequestration occurred bilaterally, next assessing whether DNA binding, reduced the activity of GA. As with LPS, membrane perturbation assays were employed, after incubating GA-DNA together at a CF-physiological concentration of eDNA (1 mg/mL). Unlike LPS, no significant differences were seen in the ability of GA to perturb the OM, CM and CE of P. aeruginosa strains PAO1, PA14 and PAK in these assays (Fig. 3b–d. Supplementary Figs. 2d, 3d, 4d).
Modification of P. aeruginosa Lipid A of type strains after GA and AMP exposure
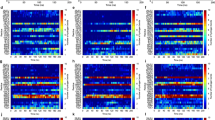
Having identified the interaction of GA and LPS and in the knowledge that many AMPs target the Lipid A component of LPS for membrane attachment, we next tested the reaction of P. aeruginosa to the targeting of its LPS by GA. P. aeruginosa has been shown to react to attack by other AMPs by sensing the peptides via TCSs and modifying the Lipid A portion of its LPS structures, reducing the charge of its CE and vulnerability to cationic AMPs. MALDI-TOF mass spectrometry was used to investigate if exposure to GA induces changes in the Lipid A structures, as Lipid A modification can result in resistance to AMPs69. The MALDI-TOF spectra of the strains PAO1, PA14 and PAK were acquired with and without exposure to AMPs (GA, CST and LL-37) and abundances of each Lipid A modification type (additions of phosphate, C10:3OH groups, palmitate and L-Ara4N) were normalised to native Lipid A, by ratio. No significant differences were seen in any modification of Lipid A across the P. aeruginosa type strains after GA exposure, when compared to the cultures without GA stress (Fig. 4). LL-37, as a positive inducer of LPS modification, showed the highest (but non-significant) addition of C10-3OH and L-Ara4N in the 3 strains70. No significant changes in LPS Lipid A modification resulted from CST exposure in the CST-sensitive type strains.
Heatmap of ratios of Native Lipid A:Modified Lipid A for each modification type. No significant differences from No Treatment (NT) were seen for any modification type as the result of any AMP tested; 50 mg/L GA, 0.5 mg/L CST or 16 mg/L LL-37 (Friedman test with Dunn’s multiple comparison). Median values of triplicate biological replicates, except LL-37 which was run in duplicate due to resource limitation. Full modifications values can be found in Supplementary Material.
Modification of P. aeruginosa Lipid A of clinical strains from people with CF after GA exposure
We next investigated changes in the Lipid A structures of 11 clinical P. aeruginosa isolates from people with CF, after the bacteria were exposed to GA, determining their lipid A profiles with and without treatment with GA and using clinical isolates which had already been characterised for GA-antibiotic synergy51. As previously, each modification type was examined as a ratio with native Lipid A and GA-exposed cultures compared to untreated. Clinical respiratory strains from people with CF showed no significant changes for any modification type of Lipid A (Fig. 5). Our interest was in investigating the response of clinical CF P. aeruginosa isolates to GA as a group, rather than identifying specific responses in individual strains and we, therefore, refrain from commenting on the (non-significant) results seen in any individual clinical isolate.
Heatmap of the ratios of Modified Lipid A:Native Lipid A for each modification type. No significant differences resulted from exposure to 50 mg/L GA, from No Treatment, for any modification type across the 11 clinical strains (Wilcoxon test). Median values of triplicate biological replicates. *GA_899 biological duplicates displayed as one replicate lost for technical reasons. Median value of biological replicates displayed. Full modifications values can be found in Supplementary Material.
Expression of TCS and arn operon genes of P. aeruginosa type strains after GA and AMP exposure
In the absence of significant LPS Lipid A modification increases in response to GA exposure and for more detail on the bacterial response to GA, the expression of the response regulator genes of TCSs (phoP, pmrA, cprR and parR) frequently associated with AMP detection as well as a gene involved in the LPS L-Ara4N modification process (arnB) were investigated using qRT-PCT34,71,72,73. The effect of exposure of the P. aeruginosa type strains to GA was once again compared to that of AMPs CST and LL-37. No significant differences were seen in the expression of any of the TCS genes, which had been normalised to expression of the housekeeping gene rpsL (ΔCt), with any intervention (Supplementary Data. Supplementary Fig. 6). For the ΔΔCt, changes compared to untreated cultures, exposure to LL-37 resulted in expression increases >2-fold of the TCS genes phoP (4.06 [95%CI 1.26–4.20]) and pmrA (5.08 [95%CI 1.91–9.13]) and the modification gene arnB (8.55 [95%CI 4.29–11.54]). Exposure to GA and CST did not result in a ΔΔCt of >2-fold increase for any of the genes tested in the type P. aeruginosa strains (each of which is sensitive to CST) (Fig. 6). These results are in keeping with the above Lipid A modification data where only LL-37 stimulates any response from the type P. aeruginosa strains.
ΔΔCt results of gene expression, normalised to untreated P. aeruginosa. Only exposure to LL-37 resulted in fold increases in gene expression >2 with median expression of phoP of 4.06 (95%CI 1.26–4.20), pmrA of 5.08 (95%CI 1.91–9.13) and arnB of 8.55 (95%CI 4.29–11.54). Each point is the median of biological replicates for P. aeruginosa PAO1 (◯), PA14 (☐) or PAK (△). Line at median. Gene expression values can be found in Supplementary Material.
Expression of TCS and arn operon genes of clinical P. aeruginosa from people with CF after GA exposure
Clinical P. aeruginosa isolates from people with CF were next tested for their reaction to GA exposure using the same set of genes as the type strains. Exposure to GA resulted in a significant increase in the expression of genes pmrA (p < 0.05) and arnB (p < 0.01) compared with untreated cultures (Supplementary Data. Supplementary Fig. 7). However, only a modest (<2-fold) median ΔΔCt change was seen for each (pmrA of 1.39 [95%CI 0.82–2.52] and arnB of 1.55 [95%CI 1.16–2.08]) (Fig. 7a). Change in expression of these two genes after exposure to 50 mg/L GA was also positively correlated (p < 0.005, Spearman r = 0.8 [95%CI 0.36–0.95]) (Fig. 7b).
a ΔΔCt results of gene expression, normalised to untreated P. aeruginosa for each strain. No gene tested had >2-fold (dotted line) median increase across the 11 clinical P. aeruginosa tested. On each graph, each point is the median of biological replicates for a clinical P. aeruginosa strain, solid line at median. b Correlation analysis of ΔΔCt expression levels of pmrA and arnB. Across the clinical strains tested, there was significant positive correlation between the expression of pmrA and arnB after exposure to GA (p = 0.0047, Spearman r = 0.8 [95%CI 0.36–0.95]. n = 11). Strain colour coding and gene expression values can be found in Supplementary Material.
The remaining genes tested were not significantly altered by GA in clinical P. aeruginosa, when compared to untreated cultures, and each had expression fold changes <2; phoP 1.01 (95%CI 0.62–1.83), cprR 1.07 (95%CI 0.48–2.00) and parR 1.01 (95%CI 0.36–1.50) (Fig. 7a). No correlation was seen in the expression levels for any of these TCS genes with expression of arnB after exposure of the clinical P. aeruginosa strains to GA. As with Lipid A modification in the same clinical isolates, our interest was in testing the overall reaction of P. aeruginosa to GA rather than identifying individual strains which gave a particular response. Therefore, despite individual strains showing >2-fold increased expression for specific gene, these reactions were in the minority across all genes tested and we do not comment on the results for any individual strain as the median response for the group was not sufficiently great.
Resistance to CST and other AMPs has been associated with specific, non-synonymous mutations in TCS genes; in true resistance these SNPs frequently lead to inactivation of the sensor genes of TCS(s) which results in constitutive activation of the L-Ara4N modification system encoded by the Arn operon74. To confirm that TCS genes from the 11 clinical strains tested were not identical, the sequences examined for SNPs resulting in amino acid changes. All clinical isolates had at least one amino acid (aa) change to one of the TCS genes examined (compared with PAO1). A total of 24 aa changes were identified in clinical isolates TCS genes ranging from 1 to 11 (Table 1. Supplementary Table 1). The strain with the highest number of aa changes (GA_899) had aa changes in the sequence of PmrB which have been associated with an intermediate level of CST resistance in clinical P. aeruginosa previously but gene expression was <2-fold for all genes tested in this strain75. (Expression of the ColSR system was not measured in this study and no amino acid changes were found in any strain in this system).
Discussion
The evidence from our previous work was that GA acts on P. aeruginosa inner and outer cell membranes, damaging both and permeabilising the cell, and it was therefore of interest to test whether electrostatic interactions with LPS were the point of contact between GA and the bacterial cell, as seen with many other cationic AMPs50,51,76,77. Here we have confirmed the binding of GA and P. aeruginosa LPS to each other via both LPS quantification and GA activity. GA sequesters and neutralises P. aeruginosa LPS and, conversely, LPS can sequester GA. These results indicate that the LPS on the surface of P. aeruginosa is a cellular target for GA, via its cationic properties allowing binding to the bacterial cell and resulting in its membrane perturbing effects.
With this information it was therefore necessary to investigate the significance for GA activity of GA-LPS interactions, particularly given the salience of free-LPS in CF sputum39,41,61,78. The results here for membrane perturbation assays further demonstrate interaction between GA and P. aeruginosa LPS; the presence of a supraphysiological concentration of LPS significantly reduced GA disruption of the OM and depolarisation of the CM. However, a CF physiological LPS concentration (0.02 mg/mL) has less impact: across the three membrane assays and the three P. aeruginosa strains tested, only OM disruption was reduced. Divalent cations of Mg2+ were also protective against previously reported OM disruption and CE permeabilisation activities of GA51. This is further confirmation of our earlier observation of GA and LPS binding and of competition between GA and Mg2+ for LPS binding, which indicates that the interaction is driven by their opposing charges, a feature of AMP activity31. While we recognise testing these elements in isolation of each other may not reflect the full complexity of the CF lung environment – which may be more detrimental to GA activity than each element alone–the elements tested here are also known to interact with and/or sequester each other and may exert a less confounding effect on GA due to their competitive binding with each other31,46.
We note that the protective effect of Mg2+ cations against GA activity was seen for the OM and CE, but absent for the CM. This may indicate that the activity of GA against P. aeruginosa cells is multimodal rather than via one distinct mechanism. GA is a random peptide with huge heterogeneity (formed of up to 1030 possible peptides), has the ability to adopt more than one conformation in solution and to oligomerise, therefore evidence of activity against the bacterial cell via more than one mechanism is perhaps not surprising54,55,79.
We were also interested in investigating any interactions between GA and DNA. As with LPS, eDNA is an important component of CF sputum; it is negatively charged, important in P. aeruginosa infection and has been shown to be a factor in AMP resistance in bacteria46,47,68,80. There were also previous indications of DNA aggregation by GA50. In common with our observations for LPS, here we have shown that there is direct interaction between GA and DNA; quantifiable DNA (1 and 10 mg/mL) was reduced by GA in a dose-dependent manner, significantly so at GA ≥ 25 mg/L. At a physiologically relevant concentration of DNA, the activity of GA was not impaired81.
With confirmation of GA interactions with LPS as a mechanism of GA activity, this leads to the question of whether or not P. aeruginosa strains would mount a defence against the targeting of its LPS by GA. Resistance to AMPs, such as CST, is associated with the modification of the Lipid A portion of LPS; the addition of positively charged moieties of various kinds to neutralise the charge of the bacterial cell and limit AMP binding82,83,84. The modification of the Lipid A component of LPS was tested using MALDI-TOF analysis on which we saw no significant changes in any of the Lipid A additions tested across the type P. aeruginosa strains. These results show that GA exposure does not result in a significant response being mounted by the type strains in response to GA activity, in the manner commonly described for other AMPs. For further details on the reaction to AMP exposures, the expression of genes involved in TCSs known to detect AMPs in P. aeruginosa (phoPQ, pmrAB, cprSR and parSR) and a gene of the arn operon were tested. These TCSs feed information to the Arn operon which controls the modification of LPS with L-Ara4N, as protection against AMP attack. We found no significant changes in gene expression levels across the type strains of P. aeruginosa due to AMP exposure, including GA. Only the human cathelicidin LL-37 resulted in median ΔΔCt >2-fold across the type strains which was the case for phoP, pmrA and arnB.
In clinical P. aeruginosa strains, no significant increases in Lipid A additions were seen after GA exposure. Expression of genes pmrA and arnB were increased in clinical strains due to GA exposure, but in neither case >2-fold greater than untreated bacteria. There was a correlation between the expression of the two significantly increased genes indicating that, even at the low level of the effect recorded, sensing of and reaction to GA by clinical P. aeruginosa is taking place via the well documented cascade of PmrAB to the Arn operon36,75. This suggests that the clinical P. aeruginosa did not fail to detect the presence of GA even if this did not lead to a notable fold increase in gene expression nor Lipid A modification by addition of L-Ara4N in clinical strains. This is despite L-Ara4N addition being the process which the Arn operon mediates. Neither was an increase in any of the other Lipid A modification types tested here seen due to GA exposure.
The search for solutions to the global AMR crisis has resulted in a renewed and increased interest in the utility of AMPs20. While issues of cytotoxicity at effective, antibacterial doses has limited the number of AMPs transferring to the clinic so far – unlikely to be an issue for the already clinically used GA–many AMPs are under investigation as new antimicrobials and AMPs have several advantages over conventional antibiotics and other novel therapies7,8,20. Chief among these is the low rate of resistance generation resulting from AMP exposure; AMPs have been shown to be less likely to produce resistance, to result in a lower recombination rate than antibiotics and CST resistance took longer to emerge than resistance to other antibiotics24,33,85. While concerns have been raised about cross-resistance between AMPs–due to their similar mechanisms of action–we did not note any crossover between the reaction of clinical P. aeruginosa to GA and their CST-sensitivity phenotypes, in our short term exposures86. The clinical strain with the strongest reaction to GA in its LPS modification (GA_899) was designated CST-sensitive clinically and the CST-resistant strain in the panel (GA_422) did not mount a strong LPS modification reaction to GA exposure. Encouragingly, overall the results presented here for GA indicate that P. aeruginosa does not mount a strong response to GA activity in a manner seen for exposure to other AMPs.
Moreover, our previous work proposes a role for GA less as a direct acting antibiotic and more as an antibiotic adjuvant to be given in combination with the aminoglycoside tobramycin, with which we have demonstrated substantial synergy51. The non-bactericidal nature of GA at a synergistic concentration and the absence of a resistance response in the results presented here both suggest reduced selective pressures for AMR development by P. aeruginosa strains in response to GA exposure. As mentioned, GA is a random peptide and random peptides are currently being researched as “resistance proof” antimicrobials with synergy between AMPs being well documented and resistance evolution having been shown to be less common for AMP combinations/cocktails than single AMPs or conventional antibiotics87,88,89,90,91. A co-treatment strategy with a conventional antibiotic further alleviates concerns for the future generation of resistance with combinations and synergy less likely to result in AMR and loss of utility (while also rescuing the efficacy of tobramycin in the case of GA)89,92,93,94,95.
The results presented here point to further potential ancillary benefits of GA–on top of the known advantages of antibiotic synergy, little bacterial resistance response and its immunomodulatory properties–with evidence that both P. aeruginosa LPS and DNA were stably bound and sequestered by GA. As well as being highly immunogenic in infection generally, free-LPS is particularly important in CF lung infections40,96. In common with other areas of the body, LPS is pro-inflammatory in CF sputum and contributes to NETosis, a key driver of cycles of inflammation and bacterial infection in CF43,44,45. Free-LPS has also been shown to exacerbate the CFTR defect and to contribute to longer-term lung damage97,98. NETosis can also lead to an increase in the eDNA content of CF sputum which is detrimental to the lung in a number of ways; eDNA is a vital component of P. aeruginosa biofilms, increases resistance to AMPs and contributes to sputum acidification resulting in increased AMR and viscosity47,68,80. Both LPS and DNA are also ligands for toll-like receptors (TLRs) and the pro-inflammatory processes which TLRs mediate and, therefore, neutralisation by AMPs, such as seen here for GA, could inhibit TLR activation99,100. Consequently, neutralisation of either/both of these sputum components could be of benefit in reducing inflammation and cycles within the CF lung which can promote P. aeruginosa persistence48,49,64,65,77. Both CST and LL-37 have been investigated as LPS neutralisers, to beneficial effect, as well as other AMPs48,63,64,101. Furthermore, AMP killing of bacteria has been shown to be “immunologically silent”, the coating of the bacteria cells by cationic AMPs preventing pro-inflammatory cascades normally associated with infection102,103.
The era of the highly effective small molecule modulator has resulted in great improvements in many clinical markers of CF lung disease in pwCF104,105,106. This includes a decrease in the frequency of infection-related exacerbations, lower bacterial loads in airways and greater lung microbiome diversity. However, some studies have reported continued lung microbiome dysbiosis and rebounding of P. aeruginosa bacterial loads, after an initial reduction, in pwCF on modulator therapy105,106. While extremely encouraging, modulators do not currently negate the need for new antimicrobials in CF107. Adult populations of pwCF already include a chronically infected proportion (pre-dating modulator therapy) along with increased and increasing life expectancy which will continue to require antibiotic therapies. Furthermore, the broader global issue AMR bacteria continues to be a concern108.
Gram-negative LPS is a crucial point of contact in host-pathogen interactions. LPS is detected by host immune systems as a marker of infection and LPS is targeted as a binding site for host defence AMPs but LPS and its modification also provide bacteria with a defence against antimicrobials. In CF, this is further complicated by the presence of free-LPS in the airway. Here, we have demonstrated that LPS plays a role in the activity of GA but the latter was not extensively inhibited by free-LPS when present in the background. We also showed that, in both type strains and isolates from people with CF, GA did not trigger a strong response in LPS modification by P. aeruginosa, either at the genetic or physical level, to defend its LPS against GA. This appears compatible with the fact that GA is not exerting strong selective pressure on systems frequently associated with AMP resistance. Interest in research into random peptide cocktails is increasing with the aim of producing “resistance proof” antimicrobials87,88,89. These results add to the previously published evidence for GA as an AMP with strong suitability for repurposing and with a strong, longstanding safety profile could be a forerunner of random peptides as treatments for infection.
Methods
Strains and growth conditions
P. aeruginosa type strains PAO1, PA14 and PAK and 11 clinical P. aeruginosa isolates were used in this study. The panel of clinical strains, which was assembled for a previous study, constitutes isolates from the CF Bacterial Repository at the National Heart and Lung Institute, Imperial College London from airway samples of people with CF at the Royal Brompton Hospital, London (Supplementary Table 2)51. Bacteria were stored in Microbank vials (Pro-Lab Diagnostics) at −80 °C. Isolates were grown overnight at 37 °C on LB agar (Merck) and stored for up to 1 week at 4 °C before further subculture to LB agar. Single colonies were inoculated into Mueller-Hinton broth (MHB) (Merck) and incubated overnight at 37 °C with agitation at 200 r.p.m.
LPS quantification
LPS was quantified as Endotoxin Units (EU) using a limulus amoebocyte lyase Endotoxin Quant Kit (ThermoFisher), per manufacturer’s instructions. P. aeruginosa LPS (Merck) at physiologically relevant CF concentrations of 0.01 and 0.02 mg/mL and the supraphysiological concentration of 0.1 mg/mL was incubated at 37 °C for 30 min, with and without 50 mg/L GA (Biocon), as was GA without LPS39,61,62. Samples were diluted 1:1000 in phosphate buffered saline (PBS), to bring them within the sensitivity range of the kit, before addition to the remaining kit components along with blanks and standards. Each condition was tested in triplicate. The optical density was measured at 405 nm (OD405) in FLUOstar Omega platereader (BMG Labtech). Blanked Standards were fitted using simple linear regression to create a standard curve (GraphPad Prism). Concentrations of samples in EU were interpolated from the standard curve via their blanked OD405 results. Neutralisation of LPS by GA was calculated as the EU of LPS in the presence of GA as a percentage of the EU of LPS in the absence of GA, at each LPS concentration tested, and compared to No Neutralisation (i.e. 0%).
DNA extraction and GA-DNA interactions
Bacterial DNA was extracted from strains PAO1, PA14 and PAK from ~1 × 108 CFU using NucleoSpin Microbial DNA Mini kit (Macherey-Nagel), as per manufacturer’s instructions. Purified DNA was stored at −20 °C. DNA concentrations were measured on a NanoDrop (ThermoFisher) and adjusted to 100 mg/mL. DNA from the individual strains were pooled together for use as ‘eDNA’, to provide a mixed source of DNA, and diluted to required final concentrations.
Concentrations of DNA were chosen as a CF physiologically relevant concentration (1 mg/mL) and a supraphysiological concentration (10 mg/mL)81. Each DNA concentration was combined with GA at concentrations of 0, 6.25, 12.5, 25, 50 and 100 mg/L and incubated at 37 °C for 30 min, as were DNA-free GA solutions, in PBS. Propidium iodide (PI) was added to the suspensions to a concentration of 1 µg/mL and fluorescence measured in a platereader at excitation 544 nm, emission 610 nm, in the wells of a black microtitre plate (ThermoFisher) (5 wells of each). In the absence of DNA, no differences were seen in PI fluorescence between buffer only wells and those containing the increasing GA concentrations, indicating there is no interaction between PI and GA. Neutralisation of DNA by GA was calculated as the fluorescence intensity of DNA in the presence of GA as a percentage of the fluorescence intensity of DNA in the absence of GA, at each DNA concentration tested. Each was compared to No Neutralisation at the same DNA concentration (i.e. 0%).
Conditions for membrane disruption assays
The effects of P. aeruginosa LPS on the previously reported rapid activity of GA against P. aeruginosa membranes were tested in two different modes. Firstly, a ‘Pre-incubation’ was performed to test the ability of LPS to sequester GA where LPS and GA were incubated together at 37 °C for 30 min before administration to cell suspensions. Secondly, in the ‘Background’ of the assays, to test the effect of LPS presence on administered GA activity, LPS was added to each assay buffer. LPS-only and GA-only solutions were also incubated under the same conditions. In both cases final concentrations of P. aeruginosa LPS of 0.01, 0.02 and 0.1 mg/mL and 50 mg/L GA were used (as above). To further test for GA-LPS interactions, the protective effect of Mg2+ cations were added to assay buffers as MgSO4 (Merck) at CF physiologically relevant levels (0.5 and 1 mM)66,67. Finally, to test for GA sequestration by DNA, 50 mg/L GA and 1 mg/mL DNA were incubated together at 37 °C for 30 min before administration to cell suspensions. DNA-only and GA-only solutions were also incubated under the same conditions. Assay specific buffers (see below) were used in each case.
Outer membrane disruption
Disruption of the bacterial OM of P. aeruginosa was measured using the fluorescent probe 1-N-Phenylnaphthylamine (NPN) (Merck) at a final concentration 10 µM76. Late-exponential phase cultures of P. aeruginosa were washed twice with 5 mM HEPES and adjusted to OD600 0.5 in appropriate buffer depending on whether a Background or Pre-incubation experiment was required. Conditions were tested as outlined above. Controls included dye free wells, GA-only treated bacteria, wells without GA treatments but containing LPS/DNA/Mg2+. Triplicate technical repeats were carried out and averaged for each biological replicate performed. Assays were performed in black microtitre plates (ThermoFisher) in 200 µL volumes and fluorescence was measured in a platereader at excitation 355 nm, emission 460 nm every 30 s for 15 min. Mean NPN Uptake Factor over 15 min was calculated as ([Fluorescence of Sample with NPN – Fluorescence of Sample without NPN]/[Fluorescence of buffer with NPN—Fluorescence of buffer without NPN]). At least three biological replicates were performed on each P. aeruginosa type strain. Comparisons to GA activity were performed separately for each element tested (LPS or divalent cations or DNA).
Cytoplasmic membrane depolarisation
Depolarisation of the CM of P. aeruginosa isolates was measured using the fluorescent probe 3,3’-Dipropylthiadicarbocyanine Iodide (DiSC3(5)) (Thermo Scientific)109. Late-exponential phase cultures of P. aeruginosa were washed twice and adjusted to an OD600 of 0.05 in 5 mM HEPES-20 mM glucose. DiSC3(5) was added to the bacterial cultures to a concentration of 1 µM and aliquoted to the wells of a black microtitre plate in 200 µL volumes with triplicate technical repeats. The fluorescent signal of the dye was allowed to quench for 30 min in the dark before addition of test solutions. Conditions were tested as outlined above. Controls included dye free wells, GA-only treated bacteria, wells without GA treatments but containing LPS/DNA/Mg2+. Fluorescence was measured in a platereader at excitation 544 nm, emission 620 nm every 30 s for 15 min. Fluorescent signals of samples were normalised to a cell free background with identical components and averaged. At least three biological replicates were performed on each P. aeruginosa type strain. Comparisons to GA activity were performed separately for each element tested (LPS or divalent cations or DNA).
Cell envelope permeability
Permeabilisation of the P. aeruginosa CE was measured using the fluorescent dye PI as per manufacturer’s instructions (Merck, UK). Late-exponential phase cultures of P. aeruginosa were washed twice and adjusted to an OD600 of 0.5 in PBS. PI was added to the bacterial cultures to a concentration of 1 µg/mL. Conditions were tested as outlined above. Fluorescence was measured in a platereader at excitation 544 nm, emission 610 nm every 30 s for 1 h in the wells of a black microtitre plate in 200 µL volumes. Technical triplicates were performed, blanked and signal averaged in each biological experiment. Area under the curve (AUC) of the PI fluorescence intensity was computed using the trapezoid rule over 1 h (GraphPad Prism). At least three biological replicates were performed on each P. aeruginosa type strain. Comparisons to GA activity were performed separately for each element tested (LPS or divalent cations or DNA).
AMP exposure
Overnight cultures of P. aeruginosa grown in MHB were centrifuged (3500 g, 15 min), supernatant discarded, bacterial pellets resuspended in fresh media and cultures adjusted to OD600 0.05 (5 × 106 CFU/mL) in MHB. AMPs were added to P. aeruginosa type strain cultures at the following final concentrations; 50 mg/L GA, 0.5 mg/L CST or 16 mg/L LL-37, along with untreated cultures. Clinical strains were incubated with No Treatment or 50 mg/L GA. After AMP addition, cultures were incubated at 37 °C with agitation at 200 r.p.m. and bacteria allowed to grow to mid-log phase (~4 h). Triplicate biological replicates of each strain at each condition were performed with the exception of LL-37 exposure, which was performed in duplicate due to resource limitation.
After incubation, OD600 was measured and ~1 × 108 CFU were harvested and placed in a 1.5 mL eppendorf and centrifuged at 15,000 g for 10 min. The supernatant was removed and the pellet was used for RNA extraction and gene expression (details below). The remainder of each culture was centrifuged (3500 g, 15 min), supernatant discarded and pellet resuspended in 1 mL sterile PBS used for MALDI-TOF mass spec analysis of Lipid A (details below).
Gene expression
Bacterial RNA was extracted from ~1 × 108 CFU after AMP exposure (above) using Direct-zol RNA Miniprep kit (Zymo) as per manufacturer’s instructions. Expression levels of TCS response regulator genes phoP, pmrA, cprR, parR, Arn operon gene arnB and housekeeping gene rpsL were measured using primers from Lee et al.34 (ThermoFisher)34. Quantitative Real-Time PCR was performed using KAPA SYBR FAST One-Step kit (KAPA) on a QuantStudio 7 Flex (ThermoFisher) with the following cycling conditions; 5 min at 42 °C for Reverse Transcription, 3 min at 95 °C for Enzyme Activation followed by 40 cycles of 10 s at 95 °C (Denaturation) and 30 s at 60 °C (Annealing/Extension). Triplicate wells of each PCR were performed as technical replicates. Cycle thresholds (Ct) for each well were calculated by the QuantStudio analysis software (ThermoFisher) and averaged. Expression of each gene was normailised to the expression of rpsL (ΔCt) and relative expression of each gene under AMP stimulation was normailised to its expression under No Treatment (ΔΔCt).
MALDI-TOF
Analysis of Lipid A conformations were carried out as previously described84. Briefly, bacteria were centrifuged at 17,000 × g for 2 min, supernatant discarded and the pellet was washed three times with 300 μL of ultrapure water and resuspended to a density of McFarland 20 as measured using a McFarland Tube Densitometer followed by acetic acid hydrolysis 1% at final concentration at 98 °C for 1 h. After 2 washes with ultrapure water, the hydrolysate was suspended in 50 μL and a volume of 0.4 μL of this suspension was loaded onto the MALDI target plate overlaid with 1.2 μL of Norharmane matrix (Sigma-Aldrich) solubilised in chloroform/methanol (90:10 v/v) to a final concentration of 10 mg/mL.
The samples were loaded onto a disposable MSP 96 target polished steel BC (Bruker Part-No. 8280800). The bacterial suspension and matrix were mixed directly on the target by pipetting. The spectra were recorded in the linear negative-ion mode (laser intensity 95%, ion source 1 = 10.00 kV, ion source 2 = 8.98 kV, lens = 3.00 kV, detector voltage = 2652 V, pulsed ion extraction = 150 ns). Each spectrum corresponded to ion accumulation of 5000 laser shots randomly distributed on the spot. The spectra obtained were processed with default parameters using FlexAnalysis v.3.4 software (Bruker Daltonik).
For each biological replicate performed, the abundance of native and modified Lipid A were enumerated via the AUCs of their spectra at each mass to charge ratio (m/z) where each appears (Supplementary Table 3). Using the total values across all m/z where each modification is seen, the ratio Native:Modified was calculated for each modification type (phosphate, C10:3OH, palmitate and L-Ara4N). One biological replicate of strain GA899 was lost during processing and subsequent data for this strain is therefore for biological duplicates.
Genomics
DNA extractions for genomic sequencing were performed using Maxwell™ RSC Cell DNA Purification Kit and automated extraction on the Maxwell RSC 48 Instrument (Promega). The DNA concentration was evaluated using a Qubit dsDNA BR assay kit and Qubit Fluorometer (ThermoFisher). Sequencing was on a standard Illumina HiSeq platform.
Genomes were assembled with Shovill and annotated using Prokka with quality control by Quast on the Galaxy platform110,111,112,113,114,115,116,117,118. The sequences of the genes of TCSs PhoPQ, PmrAB, CprSR, ParSR and ColSR of PAO1 were taken from https://pseudomonas.com/ and used to extract their corresponding sequences from the genomic data of the 11 clinical P. aeruginosa using the MyDbFinder tool (https://cge.food.dtu.dk/services/MyDbFinder/)119. Gene sequences from clinical strains were aligned with those from PAO1 using MEGA X and non-synonymous SNPs identified120. Gene accession numbers can be found in Supplementary Table 1.
Statistics
Unpaired, non-parametric data were analysed using analysis of variation (ANOVA) Kruskal-Wallis method with Dunn’s multiple correction for non-parametric (adjusted p values reported). Membrane assay data biological replicates were log transformed and compared with Welch’s ANOVA with Dunnett’s T3 for multiple comparisons and with Welch’s t test for two datasets. Paired comparisons of multiple non-parametric datasets used Friedman ANOVA test method, with Dunn’s multiple correction (adjusted p values reported). Paired comparisons of two non-parametric datasets used Wilcoxon tests. A significant difference was reported with p < 0.05. Biological replicates comprise means of technical replicates. All analyses and data presentation were performed in GraphPad Prism version 9.0 or later.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Gene accession numbers can be found in Supplementary Table 3.
References
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 399, 606–607 (2022).
Fowler, V. G., Jezek, A., Spivak, E. S. & Talkington, K. Urgent, Comprehensive Federal Action Needed To Stem Mortality and Medicare Costs Associated With Antimicrobial Resistance. Clin. Infect. Dis. 74, 1107–1111 (2021).
O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations: the review on antimicrobial resistance. 1–35 https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (2019).
Mestrovic, T. et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Heal. https://doi.org/10.1016/S2468-2667(22)00225-0 (2022).
Laws, M., Shaaban, A. & Rahman, K. M. Antibiotic resistance breakers: current approaches and future directions. FEMS Microbiol. Rev. 43, 490–516 (2019).
Browne, K. et al. A new era of antibiotics: the clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 21, 1–23 (2020).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285 (2020).
Miró-Canturri, A., Ayerbe-Algaba, R. & Smani, Y. Drug repurposing for the treatment of bacterial and fungal infections. Front. Microbiol. 10, 41 (2019).
World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. WHO Bull. 13, 104–116 (2017).
Diggle, S. P. & Whiteley, M. Microbe profile: pseudomonas aeruginosa: opportunistic pathogen and lab rat. Microbiol. 166, 30–33 (2020).
Emerson, J., Rosenfeld, M., McNamara, S., Ramsey, B. & Gibson, R. L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 34, 91–100 (2002).
Nixon, G. M. et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 138, 699–704 (2001).
Heltshe, S. L. et al. Longitudinal development of initial, chronic and mucoid Pseudomonas aeruginosa infection in young children with cystic fibrosis. J. Cyst. Fibros. 17, 341–347 (2018).
Williams, H. D. & Davies, J. C. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax 67, 465–467 (2012).
Kosorok, M. R. et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr. Pulmonol. 32, 277–287 (2001).
Moriarty, T. F., Elborn, J. S. & Tunney, M. M. Effect of pH on the antimicrobial susceptibility of planktonic and biofilm-grown clinical Pseudomonas aeruginosa isolates. Br. J. Biomed. Sci. 64, 101–104 (2007).
Høiby, N., Ciofu, O. & Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 5, 1663–1674 (2010).
Elborn, J. S. Cystic fibrosis. Lancet 388, 2519–2531 (2016).
Liu, Y. et al. The revitalization of antimicrobial peptides in the resistance era. Pharmacol. Res. 163, 105276 (2021).
Lazzaro, B. P., Zasloff, M. & Rolff, J. Antimicrobial peptides: application informed by evolution. Science 368, eaau5480 (2020).
Abd, M. et al. Colistin and its role in the Era of antibiotic resistance: an extended review (2000-2019). Emerg. Microb. Infect. 9, 868–885 (2020).
Koo, H. B. & Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 111, e24122 (2019).
Rodríguez-Rojas, A., Moreno-Morales, J., Mason, A. J. & Rolff, J. Cationic antimicrobial peptides do not change recombination frequency in Escherichia coli. Biol. Lett. 14, 20180006 (2018).
Bacalum, M. & Radu, M. Cationic antimicrobial peptides cytotoxicity on mammalian cells: an analysis using therapeutic index integrative concept. Int. J. Pept. Res. Ther. 21, 47–55 (2015).
Dijksteel, G. S., Ulrich, M. M. W., Middelkoop, E. & Boekema, B. K. H. L. Review: lessons learned from clinical trials using Antimicrobial Peptides (AMPs). Front. Microbiol. 12, 287 (2021).
Zgurskaya, H. I. & Rybenkov, V. V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 1459, 5 (2019).
Peterson, A. A., Hancock, R. E. W. & McGroarty, E. J. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J. Bacteriol. 164, 1256–1261 (1985).
Hancock, R. E. W. Peptide antibiotics. Lancet 349, 418–422 (1997).
Simpson, B. W. & Trent, M. S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 17, 403–416 (2019).
Smart, M., Rajagopal, A., Liu, W. K. & Ha, B. Y. Opposing effects of cationic antimicrobial peptides and divalent cations on bacterial lipopolysaccharides. Phys. Rev. E 96, 42405 (2017).
Klein, G. & Raina, S. Regulated assembly of LPS, its structural alterations and cellular response to LPS defects. Int. J. Mol. Sci. 20, 356 (2019).
Hamel, M., Rolain, J. M. & Baron, S. A. The history of colistin resistance mechanisms in bacteria: progress and challenges. Microorganisms 9, 1–18 (2021).
Lee, J. Y. et al. Development of colistin resistance in pmrA-, phoP-, parR- and cprR-inactivated mutants of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 69, 2966–2971 (2014).
Macfarlene, E. L. A., Kwasnicka, A. & Hancock, R. E. W. Role of Pseudomonas aeruginosa Phop-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 146, 2543–2554 (2000).
McPhee, J. B. et al. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 188, 3995–4006 (2006).
Fernández, L. et al. The two-component system CprRS senses cationic peptides and triggers adaptive resistance in Pseudomonas aeruginosa independently of ParRS. Antimicrob. Agents Chemother. 56, 6212–6222 (2012).
Gutu, A. D. et al. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob. Agents Chemother. 57, 2204–2215 (2013).
Ernst, R. K. et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286, 1561–1565 (1999).
Moskowitz, S. M. & Ernst, R. K. The role of Pseudomonas lipopolysaccharide in cystic fibrosis airway infection. Subcell. Biochem. 53, 241 (2010).
Kronborg, G., Shand, G. H., Fomsgaard, A. & Høiby, N. Lipopolysaccharide is present in immune complexes isolated from sputum in patients with cystic fibrosis and chronic Pseudomonas aeruginosa lung infection. APMIS 100, 175–180 (1992).
Okshevsky, M. & Meyer, R. L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 41, 341–352 (2015).
Dwyer, M. et al. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun. 6, 765–779 (2014).
Pieterse, E., Rother, N., Yanginlar, C., Hilbrands, L. B. & van der Vlag, J. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front. Immunol. 7, 484 (2016).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Wilton, M. et al. Chelation of membrane-bound cations by extracellular DNA activates the type VI secretion system in pseudomonas aeruginosa. Infect. Immun. 84, 2355–2361 (2016).
Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 4, 21 (2013).
Warren, H. S., Kania, S. A. & Siber, G. R. Binding and neutralization of bacterial lipopolysaccharide by colistin nonapeptide. Antimicrob. Agents Chemother. 28, 107–112 (1985).
Pulido, D., Nogús, M. V., Boix, E. & Torrent, M. Lipopolysaccharide neutralization by antimicrobial peptides: A gambit in the innate host defense strategy. Journal of Innate Immunity vol. 4 327–336 (Karger Publishers, 2012).
Christiansen, S. H. et al. The immunomodulatory drug glatiramer acetate is also an effective antimicrobial agent that kills gram-negative bacteria. Sci. Rep. 7, 1–16 (2017).
Murphy, R. A. et al. Synergistic activity of repurposed peptide drug glatiramer acetate with tobramycin against cystic fibrosis Pseudomonas aeruginosa. Microbiol. Spectr. 10, e0081322 (2022).
Schrempf, W. & Ziemssen, T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun. Rev. 6, 469–475 (2007).
Carter, N. J. & Keating, G. M. Glatiramer acetate: a review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 70, 1545–1577 (2010).
Jalilian, B., Einarsson, H. B. & Vorup-Jensen, T. Glatiramer acetate in treatment of multiple sclerosis: a toolbox of random co-polymers for targeting inflammatory mechanisms of both the innate and adaptive immune system? Int. J. Mol. Sci. 13, 14579–14605 (2012).
Christiansen, S. H. et al. The random co-polymer glatiramer acetate rapidly kills primary human leukocytes through sialic-acid-dependent cell membrane damage. Biochim. Biophys. Acta - Biomembr. 1859, 425–437 (2017).
Messina, S. & Patti, F. The pharmacokinetics of glatiramer acetate for multiple sclerosis treatment. Expert Opin. Drug Metab. Toxicol. 9, 1349–1359 (2013).
Prod’homme, T. & Zamvil, S. S. The evolving mechanisms of action of glatiramer acetate. Cold Spring Harb. Perspect. Med. 9, a029249 (2019).
Jung, S. et al. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate. J. Neuroimmunol. 148, 63–73 (2004).
Vieira, P. L., Heystek, H. C., Wormmeester, J., Wierenga, E. A. & Kapsenberg, M. L. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J. Immunol. 170, 4483–4488 (2003).
Haas, J. et al. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J. Neuroimmunol. 216, 113–117 (2009).
Raoust, E. et al. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4, e7259 (2009).
Buyck, J. M. et al. P. aeruginosa LPS stimulates calcium signaling and chloride secretion via CFTR in human bronchial epithelial cells. J. Cyst. Fibros. 12, 60–67 (2013).
Scott, A. et al. Evaluation of the ability of LL-37 to neutralise LPS in vitro and Ex vivo. PLoS One 6, e26525 (2011).
Schromm, A. B. et al. Cathelicidin and PMB neutralize endotoxins by multifactorial mechanisms including LPS interaction and targeting of host cell membranes. Proc. Natl. Acad. Sci. USA 118, e2101721118 (2021).
Grassi, L. et al. The anti-microbial peptide (Lin-SB056-1)2-K reduces pro-inflammatory cytokine release through interaction with Pseudomonas aeruginosa lipopolysaccharide. Antibiotics 9, 1–14 (2020).
Kilbourn, J. P. Composition of sputum from patients with cystic. Curr. Microbiol. 11, 19–22 (1984).
Sanders, N. N. et al. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 61, 962–968 (2006).
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. Extracellular DNA required for bacterial biofilm formation. Science 295, 1487 (2002).
Bechinger, B. & Gorr, S. U. Antimicrobial peptides: mechanisms of action and resistance. J. Dent. Res. 96, 254–260 (2017).
Strempe, N. et al. Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS One 8, e82240 (2013).
Fernández, L. et al. Adaptive resistance to the ‘last hope’ antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob. Agents Chemother. 54, 3372–3382 (2010).
Zhang, H., Srinivas, S., Xu, Y., Wei, W. & Feng, Y. Genetic and biochemical mechanisms for bacterial Lipid A modifiers associated with polymyxin resistance. Trends Biochem. Sci. 44, 973–988 (2019).
Baron, S. et al. Inactivation of the arn operon and loss of aminoarabinose on lipopolysaccharide as the cause of susceptibility to colistin in an atypical clinical isolate of proteus vulgaris. Int. J. Antimicrob. Agents 51, 450–457 (2018).
Olaitan, A. O., Morand, S. & Rolain, J. M. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 5, 643 (2014).
Lee, J. Y. & Ko, K. S. Mutations and expression of PmrAB and PhoPQ related with colistin resistance in Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 78, 271–276 (2014).
Sabnis, A. et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife 10, e65836 (2021).
Xhindoli, D. et al. The human cathelicidin LL-37 - A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta - Biomembr. 1858, 546–566 (2016).
Porro, C. et al. Pro-inflammatory effect of cystic fibrosis sputum microparticles in the murine lung. J. Cyst. Fibros. 12, 721–728 (2013).
Bawa, R., Audette, G. F. & Rubinstein, I. Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy and Clinical Applications. Vol. 1 CRC Press (2016).
Sarkar, S. Release mechanisms and molecular interactions of Pseudomonas aeruginosa extracellular DNA. Appl. Microbio. Biotechnol. 104, 6549–6564 (2020).
Brandt, T., Breitenstein, S., Von Der Hardt, H. & Tümmler, B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax 50, 880–882 (1995).
Sultan, M., Arya, R. & Kim, K. K. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int. J. Mol. Sci. 22, 12152 (2021).
Jeannot, K., Bolard, A. & Plésiat, P. Resistance to polymyxins in Gram-negative organisms. Int. J. Antimicrob. Agents 49, 526–535 (2017).
Jeannot, K. et al. Detection of Colistin resistance in Pseudomonas aeruginosa using the MALDIxin test on the routine MALDI biotyper sirius mass spectrometer. Front. Microbiol. 12, 2466 (2021).
Yu, G., Baeder, D. Y., Regoes, R. R. & Rolff, J. Predicting drug resistance evolution: Insights from antimicrobial peptides and antibiotics. Proc. R. Soc. B Biol. Sci. 285, 20172687 (2018).
Fleitas, O. & Franco, O. L. Induced bacterial cross-resistance toward host antimicrobial peptides: a worrying phenomenon. Front. Microbiol. 7, 381 (2016).
Bennett, R. C. et al. Random peptide mixtures as safe and effective antimicrobials against Pseudomonas aeruginosa and MRSA in mouse models of bacteremia and pneumonia. ACS Infect. Dis. 7, 672–680 (2021).
Amso, Z. & Hayouka, Z. Antimicrobial random peptide cocktails: a new approach to fight pathogenic bacteria. Chem. Commun. 55, 2007–2014 (2019).
Maron, B., Rolff, J., Friedman, J. & Hayouka, Z. Antimicrobial peptide combination can hinder resistance evolution. Microbiol. Spectr. 10, e0097322 (2022).
Dobson, A. J., Purves, J., Kamysz, W. & Rolff, J. Comparing selection on S. aureus between antimicrobial peptides and common antibiotics. PLoS One 8, e76521 (2013).
Yu, G., Baeder, D. Y., Regoes, R. R. & Rolff, J. Combination effects of antimicrobial peptides. Antimicrob. Agents Chemother. 60, 1717–1724 (2016).
Liu, Y., Li, R., Xiao, X. & Wang, Z. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 45, 301–314 (2019).
Worthington, R. J. & Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 31, 177–184 (2013).
Langendonk, R. F., Neill, D. R. & Fothergill, J. L. The building blocks of antimicrobial resistance in Pseudomonas aeruginosa: implications for current resistance-breaking therapies. Front. Cell. Infect. Microbiol. 11, 665759 (2021).
Baym, M., Stone, L. K. & Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 351, aad3292 (2016).
Huszczynski, S. M., Lam, J. S. & Khursigara, C. M. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens 9, 6 (2020).
Bruscia, E. M. et al. Increased susceptibility of Cftr_/_ mice to LPS-induced lung remodeling. Am. J. Physiol. - Lung Cell. Mol. Physiol. 310, L711–L719 (2016).
Cho, D. Y. et al. LPS decreases CFTR open probability and mucociliary transport through generation of reactive oxygen species. Redox Biol. 43, 101998 (2021).
Scheenstra, M. R., van Harten, R. M., Veldhuizen, E. J. A., Haagsman, H. P. & Coorens, M. Cathelicidins modulate TLR-activation and inflammation. Front. Immunol. 11, 1137 (2020).
Mookherjee, N., Anderson, M. A., Haagsman, H. P. & Davidson, D. J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19, 311–332 (2020).
Matzneller, P. et al. Colistin reduces LPS-triggered inflammation in a human sepsis model in vivo: a randomized controlled trial. Clin. Pharmacol. Ther. 101, 773–781 (2017).
Coorens, M. et al. Killing of Pseudomonas aeruginosa by chicken cathelicidin-2 is immunogenically silent, preventing lung inflammation in vivo. Infect. Immun. 85, e00546–17 (2017).
Coorens, M. et al. Cathelicidins inhibit escherichia coli –induced TLR2 and TLR4 activation in a viability-dependent manner. J. Immunol. 199, 1418–1428 (2017).
Tümmler, B. Post-approval studies with the CFTR modulators Elexacaftor-Tezacaftor—Ivacaftor. Front. Pharmacol. 14, 1158207 (2023).
Boutin, S. et al. The effect of CFTR modulators on airway infection in cystic fibrosis. Int. J. Mol. Sci 2022, 3513 (2022).
Yi, B., Dalpke, A. H. & Boutin, S. Changes in the cystic fibrosis airway microbiome in response to CFTR modulator therapy. Front. Cell. Infect. Microbiol. 11, 184 (2021).
Davies, J. C. & Martin, I. New anti-pseudomonal agents for cystic fibrosis- still needed in the era of small molecule CFTR modulators? Expert Opin. Pharmacother. 19, 1327–1336 (2018).
Shoeb, M., Islam, R. & Parvin, N. Antibiotic resistance: a global threat to humanity. Transcend. Humanit. Eng. Strateg. Sustain. Futures 20, 82–105 (2023).
Hubbard, A. T. M. et al. Mechanism of action of a membrane-active quinoline-based antimicrobial on natural and model bacterial membranes. Biochemistry 56, 1163–1174 (2017).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Seeman, T. Shovill: Faster SPAdes assembly of Illumina reads. GitHub. https://github.com/tseemann/shovill (2017).
Cuccuru, G. et al. Orione, a web-based framework for NGS analysis in microbiology. Bioinformatics 30, 1928–1929 (2014).
Mikheenko, A., Prjibelski, A., Saveliev, V., Antipov, D. & Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 34, i142–i150 (2018).
Mikheenko, A., Saveliev, V. & Gurevich, A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics 32, 1088–1090 (2016).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Mikheenko, A., Valin, G., Prjibelski, A., Saveliev, V. & Gurevich, A. Icarus: visualizer for de novo assembly evaluation. Bioinformatics 32, 3321–3323 (2016).
Rhodes, K. A. & Schweizer, H. P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 28, 82–90 (2016).
Batut, B. et al. Community-driven data analysis training for biology. Cell Syst. 6, 752–758.e1 (2018).
Winsor, G. L. et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44, D646–D653 (2016).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Acknowledgements
Authors would like to thank the Quadram Institute Biosciences Core Sequencing and Core Bioinformatics teams their assistance in this project. This project was funded by CF Trust as part of a Strategic Research Centre grant and Venture Innovation Award. Sequencing and Bioinformatics in this project was funded by BBSRC Institute Strategic Programme Microbes in the Food Chain BB/R012504/1. A.S. was supported by a PhD studentship funded by a Medical Research Council Doctoral Training Award to Imperial College London (MR/N014103/1). A.M.E. acknowledges support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). L.M.N. was supported by an Imperial College Research Fellowship (ICRF) and a Cystic Fibrosis Trust Venture Innovation Award (VIA 070). The group is supported by the National Institute for Health and Care Research through the Imperial Biomedical Research Centre and a Senior Investigator Award to J.C.D.
Author information
Authors and Affiliations
Contributions
R.A.M. and J.C.D. conceived the study. R.A.M., J.P., L.C., A.S., A.M.E., L.M.N., T.V.-J., G.L.-M. and J.C.D. contributed to the conception and design of the analyses. R.A.M., J.P., L.C. and L.M.N. performed the experiments and collected data. R.A.M. and J.C.D. analysed the data. R.A.M. and J.C.D. drafted the manuscript. All authors read and contributed to revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The Authors declare the following competing interests; Cycle Pharmaceuticals provided Glatiramer Acetate as an in-kind contribution to the CF Trust grant for this work. GA is under license at the University of Aarhus, at which T.V.-J. is employed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murphy, R.A., Pizzato, J., Cuthbertson, L. et al. Antimicrobial peptide glatiramer acetate targets Pseudomonas aeruginosa lipopolysaccharides to breach membranes without altering lipopolysaccharide modification. npj Antimicrob Resist 2, 4 (2024). https://doi.org/10.1038/s44259-024-00022-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44259-024-00022-x