Abstract
Fungal pathogens are a severe public health problem. The leading causative agents of systemic fungal infections include species from the Candida, Cryptococcus, and Aspergillus genera. As opportunistic pathogens, these fungi are generally harmless in healthy hosts; however, they can cause significant morbidity and mortality in immunocompromised patients. Despite the profound impact of pathogenic fungi on global human health, the current antifungal armamentarium is limited to only three major classes of drugs, all of which face complications, including host toxicity, unfavourable pharmacokinetics, or limited spectrum of activity. Further exacerbating this issue is the growing prevalence of antifungal-resistant infections and the emergence of multidrug-resistant pathogens. In this review, we discuss the diverse strategies employed by leading fungal pathogens to evolve antifungal resistance, including drug target alterations, enhanced drug efflux, and induction of cellular stress response pathways. Such mechanisms of resistance occur through diverse genetic alterations, including point mutations, aneuploidy formation, and epigenetic changes given the significant plasticity observed in many fungal genomes. Additionally, we highlight recent literature surrounding the mechanisms governing resistance in emerging multidrug-resistant pathogens including Candida auris and Candida glabrata. Advancing our knowledge of the molecular mechanisms by which fungi adapt to the challenge of antifungal exposure is imperative for designing therapeutic strategies to tackle the emerging threat of antifungal resistance.
Similar content being viewed by others
Introduction
Fungal pathogens are a significant threat to human health, infecting billions and taking the lives of approximately 1.5 million people annually1. Patient populations most susceptible to systemic fungal infections include immunocompromised persons; including those with HIV/AIDS, and patients receiving immunosuppressive agents, or undergoing cancer therapy2. The predominant aetiological agents of systemic fungal infections are Candida, Cryptococcus, and Aspergillus species1. Among Candida species, Candida albicans is the most common cause of invasive candidiasis globally. As a typical resident of the human microbiota, C. albicans can exist as a harmless commensal in healthy individuals; however, this fungus can cause life-threatening infections in immunocompromised patients with mortality rates exceeding 40% despite treatment2. Cryptococcus is the second leading cause of mortality in adults living with HIV in sub-Saharan Africa, with an estimated global fatality of 112,000 people every year3. Most cryptococcal infections are attributed to Cryptococcus neoformans, which can be acquired by inhaling infective airborne yeast cells2. Aspergillus species are ubiquitous moulds causing invasive aspergillosis, which carries a mortality rate of 50% even with proper diagnosis and intervention, and if left untreated, fatality can approach 100%1. Like Cryptococcus, the primary route of Aspergillus transmission is through inhalation of airborne spores, causing severe illnesses in people with weakened immune systems2. The primary causative agent of invasive aspergillosis is Aspergillus fumigatus, an organism naturally found in the soil as a saprotroph on organic debris2. Thus, the alarming impact of opportunistic fungi on global public health solicits a pressing need for effective therapeutic strategies to combat these life-threatening infections.
The generation of antifungals has been slow-paced in part due to the close evolutionary relationship between fungi and humans, limiting the number of fungal-specific targets that can be exploited for drug development. Indeed, only three major classes of antifungal drugs exist in clinical practice to treat invasive fungal diseases (Fig. 1). The polyenes (e.g., amphotericin B) represent the oldest antifungal drugs used in medical practice to treat systemic fungal infections. These amphipathic molecules exert fungicidal activity by extracting ergosterol from lipid bilayers, akin to a “sterol sponge”4. While polyenes exhibit broad-spectrum bioactivity against yeast and mould species, their significant host toxicity disfavours their clinical use5. However, amphotericin B remains the treatment of choice in resource-limited settings, particularly for the treatment of infections caused by C. neoformans. Specifically, the combination regimen of amphotericin B with the antimetabolite pyrimidine analogue flucytosine is a first-line treatment for cryptococcal meningitis in HIV-infected patients6. The azoles (e.g., fluconazole) are a class of five-membered heterocyclic compounds, which have been widely deployed in the clinic for decades to treat systemic fungal infections. Like polyenes, the azoles also target the fungal cell membrane; however, azoles are fungistatic against Candida and work by inhibiting the cytochrome P450 lanosterol 14-α-demethylase (Erg11). This results in a block in ergosterol biosynthesis and the build-up of a toxic sterol produced by the Δ-5,6-desaturase (Erg3)7. While azoles are well tolerated, a significant drawback is their potential to disrupt the metabolism of other drugs, as they also inhibit mammalian cytochrome P4505. Finally, the echinocandins (e.g., caspofungin) are cyclic hexapeptides with lipid side chains that target the fungal cell wall via inhibition of (1,3)-β-D-glucan synthase. While echinocandins exert fungicidal and fungistatic activity against Candida and Aspergillus, respectively, this drug class is ineffective against C. neoformans5.
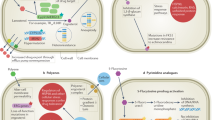
a The echinocandins function by inhibiting (1,3)-β-D-glucan synthase, disrupting cell wall integrity and causing severe cell wall stress. Echinocandin resistance is primarily mediated by mutations in the drug target gene, FKS1, in Candida, Cryptococcus, and Aspergillus. For C. glabrata, mutations occur in both FKS1 and its paralogue FKS2. Cellular factors enabling responses to echinocandin-induced stress include the molecular chaperone Hsp90, various Hsp90 client proteins, and genes that regulate cell wall salvage signalling (e.g., compensatory upregulation of chitin synthesis). b The azoles act on the fungal cell membrane via the inhibition of lanosterol 14-α-demethylase (Erg11), which blocks ergosterol biosynthesis and results in the accumulation of a toxic sterol intermediate (14-α-methyl-3,6-diol) produced by Erg3. Azole resistance can arise through mutations in the drug target (ERG11) or target overexpression. Loss-of-function mutations in ERG3 can also confer azole resistance by blocking toxic sterol accumulation and this mechanism is contingent on Hsp90 and its client proteins. Efflux is also a major azole resistance determinant and involves the upregulation of ABC and MF transporters. Aneuploidies such as a duplication of the left arm of chromosome 5 (termed isochromosome (i5(L))) can increase dosage of the azole target Erg11 and efflux pumps. c The polyene drug amphotericin B forms extramembranous aggregates that extract ergosterol from fungal cell membranes, acting as a sterol “sponge”. While resistance remains extremely rare, it can be acquired through mutations in ergosterol biosynthesis genes resulting in the depletion of ergosterol and the accumulation of alternate sterols. As with resistance to other antifungals, resistance to amphotericin B is also contingent on Hsp90-dependent stress responses.
Alarmingly, our limited repertoire of antifungal drugs is further threatened by the emergence of drug-resistant isolates in the clinic. Resistance is readily acquired through drug target alteration or overexpression, enhanced efflux pump activity, or through activation of cellular stress response pathways (Fig. 1). Further compounding this public health threat is the increased prevalence of inherently drug-resistant fungi, such as the emerging non-albicans Candida species, Candida auris and Candida glabrata8. Thus, in light of the growing public health threat of drug-resistant pathogenic fungi, a thorough understanding of antifungal resistance mechanisms is vital for the management of infections caused by these microbes. Here, we summarize our current knowledge of the molecular mechanisms underlying resistance in major and emerging fungal pathogens.
Drug target alteration or overexpression
Drug target alteration is a common mechanism leading to acquired antifungal resistance. For azoles, resistance involves alterations in genes encoding the azole drug target 14-α-demethylase (ERG11 in yeast and cyp51 in moulds). In clinical isolates of C. albicans, over 140 distinct Erg11 amino acid substitutions have been reported in the literature, with most clustering into three hot spot regions9,10. Structural analysis of C. albicans Erg11 demonstrated that while some substitutions involve residues that are exposed in the active-site cavity (directly affecting azole binding), others occur on the proximal surface of the enzyme and may indirectly contribute to azole resistance (for example, by influencing interactions with cytochrome P450 reductase)11. Mutations in ERG11 also contribute to reduced azole susceptibility in the emerging fungal pathogen C. auris12,13,14,15, with a recent study highlighting a previously unreported substitution in Erg11 (F444L) identified in azole-resistant clade IV clinical isolates that has not been previously described in other reports15. For C. neoformans, a limited number of ERG11 mutations have been described in azole-resistant clinical isolates16. Unlike Candida or Cryptococcus, A. fumigatus expresses two genes encoding Cyp51 isoenzymes, namely cyp51A and cyp51B, that are 60% identical. Mutation of cyp51A is a well-characterized and frequently-documented determinant for triazole resistance in A. fumigatus, however, the role of cyp51B mutations in azole resistance is less clear17,18. Recently, it was demonstrated that introduction of a G457S Cyp51B substitution, originally identified in a resistant clinical isolate, into a susceptible recipient conferred voriconazole resistance, reinforcing the notion that mutations in cyp51B should be monitored in the context of clinical resistance17. Alarmingly, duplications in the cyp51A gene promoter coupled with specific amino acid substitutions, TR34/L98H and TR46/Y121F/T289A, are becoming widespread in the environment associated with azole use in agriculture, while also isolated from hospital patients without previous exposure to antifungal drugs19,20,21,22. Recent genomic analysis of A. fumigatus isolates from the United States suggests a single introduction of a strain with the TR34/L98H substitution resulted in widespread distribution throughout susceptible Aspergillus populations through recombination between resistant and susceptible isolates23. Collectively, this growing body of literature suggests that environmental Aspergillus isolates serve as a reservoir for drug-resistant clinical infections around the globe.
Increased drug target expression is another prevalent mode of acquiring azole resistance. In azole-resistant clinical isolates of C. albicans, gain-of-function mutations in the zinc cluster transcription factor gene UPC2 can lead to constitutive overexpression of ERG1124. Upc2 contains an N-terminal nuclear localization signal (NLS) and a C-terminal ligand-binding domain (LBD), the latter of which can sense cellular ergosterol levels25. The Upc2 LBD exhibits ligand-dependent conformational switching and mutations of the glycine-rich loop interfere with ligand binding via steric clashes, resulting in constitutive activation26. Intriguingly, recent work demonstrated an expansive role of C. glabrata Upc2A (C. glabrata homologue of C. albicans Upc2) in controlling the expression of genes beyond those involved in ergosterol biosynthesis, including those associated with translation and plasma membrane constituents27. While the roles of these target genes in mediating antifungal resistance remains enigmatic, it would be interesting to directly assess their impact(s) on azole susceptibility. In A. fumigatus and C. neoformans, expression of cyp51A /ERG11 in response to azole exposure is regulated by the sterol regulatory element-binding protein (SREBP), SrbA and Sre1, respectively28,29. Additional regulators of cyp51A expression that contribute to azole resistance in A. fumigatus include the fungal-specific transcription factors AtrR and SltA30,31,32.
As with azoles, resistance to echinocandin therapy frequently involves mutations in genes encoding the drug target, FKS1 (Candida, Cryptococcus, and Aspergillus spp.) and FKS2 (C. glabrata only)33,34. In C. albicans, FKS1 is an essential gene encoding a catalytic subunit of the 1,3-β-glucan synthase complex, whereas in C. glabrata, FKS1 and FKS2 are functionally redundant for viability35. A recent study leveraging experimental evolution with C. glabrata generated 121 anidulafungin-resistant lineages. Intriguingly, all 121 strains harboured non-synonymous mutations in FKS genes, with mutations preferentially occurring in FKS2 as opposed to FKS136. Although most mutations were identified in well-characterized hot-spot regions, 22% of FKS mutations were identified elsewhere in the open reading frame, highlighting the importance of monitoring the contribution of mutations across the entirety of the FKS genes as opposed to simply focusing on hot-spot regions. Several amino acid substitutions in FKS1 have also been associated with decreased echinocandin susceptibility in C. auris12,37,38,39,40,41,42. Recently, through the use of cryo-electron microscopy with the model yeast Saccharomyces cerevisiae, structural analysis of echinocandin-resistant substitutions in Fks1 indicated three hot spot regions clustered at a region near transmembrane (TM) helices 5–6 and 8 which is surrounded by ordered lipid molecules43. Further analysis of the structure of mutant Fks1 (S643P), which affects lipid binding, revealed conformational changes that lead to shifts of nearby bound lipids, suggesting a potential additional mechanism for drug resistance that does not simply result in a decrease in drug binding to its target but that is a result of alterations in cellular physiology43. Additionally, the authors developed a radiolabel-free assay system for Fks1 activity, whereby activity is measured by the release of UDP. Together, this work lays the framework for future analyses of the structural implications of FKS1 mutations in other fungal pathogens and provides a platform for the development of more effective inhibitors of this important antifungal target.
For A. fumigatus, echinocandin resistance in clinical settings is extremely rare, likely due to the fitness cost of FKS1 mutations in vivo44. In a mouse model of invasive pulmonary aspergillosis, infection with an echinocandin-resistant strain of A. fumigatus was less lethal when compared with the echinocandin-susceptible strain, as demonstrated by reduced lung fungal burden and significantly longer survival time44. Notably, echinocandins retained some activity against the A. fumigatus FKS1 mutant strain in vivo, albeit a lack of appreciable activity in vitro, which may further contribute to the rare clinical occurrence of echinocandin resistance in this species44.
Severe fitness trade-offs disfavour the acquisition of resistance to the polyene amphotericin B in multiple fungal species, explaining why resistance to this antifungal class remains extremely limited in the laboratory and clinic45. Resistance may arise from alterations in the ergosterol biosynthesis pathway, causing depletion of ergosterol and the accumulation of alternate sterols. In Candida species, amphotericin B resistance has been associated with mutations in several ergosterol biosynthesis genes, including ERG2, ERG3, ERG6, and ERG1142,45,46,47. However, such mutations that confer polyene resistance simultaneously create severe cellular stress, which results in hypersensitivity to oxidative stress, febrile temperatures, and killing by neutrophils45. In C. neoformans, despite the use of amphotericin B as the first line of defence against meningeal cryptococcosis, only one case of a resistant clinical isolate harbouring a mutation in ERG2 has been documented to date48. Overall, target alteration is a canonical mechanism by which fungal pathogens evolve resistance, particularly to the azoles and echinocandins, representing a significant clinical challenge to antifungal therapy.
Increased efflux
In addition to target alteration or overexpression, enhanced drug efflux is a dominant mechanism of resistance to diverse antimicrobials. Resistance to azole therapy often involves the upregulation of plasma membrane efflux pumps, which act to reduce intracellular drug accumulation. In contrast, efflux-mediated resistance mechanisms are not involved in adaptive resistance to echinocandins or polyenes, as they are poor pump substrates49. For C. albicans, upregulation of the ATP-binding cassette (ABC) transporters, Cdr1 and Cdr2 (Candida drug resistance 1 and 2), and major facilitator transporter Mdr1 (multidrug resistance 1) have been implicated in azole resistance50,51. Specifically, gain-of-function mutations in the transcriptional activator gene, TAC1, result in constitutive overexpression of both CDR1 and CDR2, while activating mutations in MRR1 are responsible for the upregulation of MDR152,53. Likewise, gain-of-function mutations in TAC1B (C. auris homologue of C. albicans TAC1) and MRR1A (C. auris homologue of C. albicans MRR1) contribute to clinical fluconazole resistance in C. auris54,55. For example, a study examining 304 globally-distributed C. auris clinical isolates representing each of four of the major genetic clades identified three predominant TAC1B mutations, encoding A640V, A657V, and F862_N866del substitutions, from clades Ic, Ib, and IV, respectively54. Additional work demonstrated another TAC1B mutation (encoding S611P) capable of conferring enhanced fluconazole resistance among clade-IV clinical isolates, further highlighting that TAC1B mutations contribute to azole resistance across diverse clades15. In contrast, mutations in MRR1A appear to play a more restrictive role with resistance through an N647T substitution, which is reported specifically in clade III isolates56. In C. glabrata, the expression of CDR1 is controlled by the zinc cluster transcription factor, Pdr1 (pleiotropic drug resistance 1)57,58. Recently, it was demonstrated that the zinc cluster transcription factor Upc2A also regulates CDR1 expression and that extensive overlap exists between the regulatory networks mediated by Upc2A and Pdr1 in C. glabrata27,59. Overexpression of multidrug efflux transporters also mediates azole resistance in other fungal pathogens, with the major players being the ABC transporters Afr1 and AtrF for C. neoformans and A. fumigatus, respectively60,61. Additionally, in A. fumigatus, the fungal-specific Zn2-Cys6 type transcription factor AtrR plays a pivotal role in regulating the expression of cyp51A30,31. Thus, efflux-mediated resistance to azoles is active in diverse fungal pathogens, highlighting the potential for targeting drug efflux to overcome resistance.
Genomic plasticity
In addition to specific point mutations leading to enhanced drug target or efflux pump expression, another mechanism by which this can occur in fungal pathogens is through genomic alterations. In the diploid organism C. albicans, the duplication of the left arm of chromosome 5 to form an isochromosome (i5(L)) is observed in azole-resistant clinical isolates, as well as laboratory-derived resistant strains62. This aneuploidy results in increased dosage of genes encoding both the azole target Erg11 and the transcription factor regulating drug efflux, Tac163. Likewise, experimental evolution studies in C. auris suggest that an extra copy of chromosome 5 (which harbours several drug resistance-related genes) contributes to the development of fluconazole resistance in this pathogen42,64. Additionally, loss of heterozygosity (LOH) events can occur in specific genomic regions harbouring azole resistance determinants (ERG11 and TAC1), rendering mutations acquired in these genes homozygous and thus increasing resistance65. Strikingly, changes in whole chromosome copy number can occur in response to diverse stress conditions and enable tolerance to antifungals despite no prior exposure. Recent work in C. albicans demonstrated that exposure to the cancer chemotherapeutic hydroxyurea, or the ER stress-inducing agent tunicamycin selects for trisomy of chromosome 2, which also enables tolerance to the echinocandin caspofungin66,67. Given that aneuploidy simultaneously changes the copy number of many genes at a given time, it has the potential to confer cross-tolerance to unrelated drugs.
Another significant source of genomic diversity that drives the rapid acquisition of azole resistance in C. albicans involves extensive copy number variations (CNVs), which amplify large genomic regions68. These complex CNVs are flanked by distinct long inverted repeat sequences and are found across the genome, often containing genes associated with drug resistance68. Interestingly, recent work observed that different antifungal drug concentrations can select for distinct genotypic and phenotypic outcomes in vitro. Through the evolution of 144 C. albicans lineages in the presence of various concentrations of fluconazole, the authors observed that those lineages evolved with drug concentrations close to their MIC50 (the minimum inhibitory concentration of drug that reduces growth by 50%) had a propensity to rapidly evolve an increased MIC50 and acquired distinct segmental aneuploidies and CNVs69. In contrast, lineages evolved with drug concentrations above their MIC50 acquired diverse mutational changes and increase in drug tolerance (the ability of a subpopulation of cells to grow above their MIC50)69. Although the mechanisms driving these distinct phenotypes remain to be investigated in detail, it was proposed that cells experience a different degree of stress depending on the drug concentration. In the presence of higher drug concentrations (supra-MIC), cells may slow down cell cycle progression to maintain viability, while cells may persist through cell cycle progression when exposed to lower drug concentrations, increasing the frequency of segmental aneuploidies. Furthermore, while mechanistic investigations into aneuploidy-mediated antifungal resistance have been undertaken with strains harbouring i(5L), further work is needed to characterize the genes contributing to resistance in isolates with other aneuploidies. In addition, future studies should explore different drug concentrations when evaluating the efficacy of antifungals, as well as the initial MIC of a clinical isolate, to improve treatment outcomes.
For the haploid yeast C. glabrata, genome rearrangements underlying azole resistance include chromosomal translocations, segmental duplications, and occasionally, the formation of de novo minichromosomes70. For C. neoformans, a phenomenon referred to as heteroresistance can lead to resistant populations. Here, single cells give rise to progeny with heterogeneous resistant phenotypes, with a small but consistent subset of progeny highly resistant to the azoles. This phenomenon allows populations to adapt to increasing concentrations of the azoles in a stepwise manner, although susceptibility is typically restored after passage in the absence of drug. In C. neoformans, heteroresistance is often acquired through the formation of disomies, the most prevalent being that of chromosome 1, which contains both ERG11 and the ABC transporter gene AFR171. Beyond genome rearrangements, horizontal gene transfer (HGT)-mediated antifungal resistance was recently demonstrated in A. fumigatus72. Notably, A. fumigatus can undergo both chromosomal and plasmid-mediated HGT of voriconazole resistance (via a mutated resistance-conferring allele of cyp51A), with the latter being detected under conditions of voriconazole stress alone, implicating HGT as a potential route to antifungal resistance in this pathogen72. Most fungal HGT events have been studied using in silico analyses, which ideally require the use of multiple independent methods to achieve a total evidence approach73. The experimental system and insights provided by this work lay the groundwork for future studies investigating the mechanism(s) underlying HGT in fungi. Beyond the stressors used in this study, it will be of interest to test HGT induction in additional host-relevant conditions to explore the extent to which HGT contributes to fungal pathogenicity. Thus, fungal pathogens possess remarkable genomic plasticity that promotes survival in different environments, with significant implications for the evolution of antifungal drug resistance.
Cellular stress responses
Cellular stress response pathways are critical in enabling pathogen survival in response to diverse environmental perturbations and, as such, play integral roles in alleviating antifungal-induced stress (Fig. 2). One central cellular hub mediating antifungal drug tolerance and resistance is the molecular chaperone, Hsp90, which stabilizes various signal transducers involved in enabling antifungal-induced stress in Candida, Aspergillus, and Cryptococcus74,75. In C. albicans, key client proteins of Hsp90 include the Ca2+-calmodulin-activated protein phosphatase calcineurin, and multiple members of the PKC-MAPK cell wall integrity cascade (Pkc1, Bck1, Mkk2 and Mkc1)75,76,77,78. Consequently, Hsp90 inhibition blocks the activation of calcineurin-dependent stress responses and PKC signalling, abrogating tolerance and resistance to the azole and echinocandin drugs76,78. Moreover, the emergence of polyene resistance in Candida is also contingent on Hsp90, highlighting the conserved role of this protein in the acquisition of resistance to diverse antifungals45. Recently, the importance of Hsp90 in mediating cellular responses to azoles was also described in C. neoformans. Through the generation of a strain where levels of HSP90 could be repressed with a copper-repressible promoter, the authors showed depletion of HSP90 increases susceptibility to azoles as well as other cellular stressors in this pathogen79. While this work provides a tractable genetic system to dissect Hsp90 function in C. neoformans, it will be useful to develop alternative conditional-repression systems to improve gene regulation and bypass toxicity associated with copper treatment alone79. The vast majority of essential genes in C. neoformans have yet to be interrogated due to the technical constraints of genetic manipulation in this organism. Advancements in genetic tools to study essential gene function will propel the search for antifungal targets to control cryptococcal infection.
A global regulator of stress response circuitry is the molecular chaperone Hsp90. Hsp90 stabilizes key cell wall and membrane integrity regulators including the protein phosphatase calcineurin, PKC-MAPK pathway members, as well as other clients that remain to be identified. Hsp90 is subject to negative regulation by casein kinase 2 (CK2) and positive regulation by lysine deacetylases (KDACs). In addition to KDACs, lysine acetyltransferases such as Gcn5 have been implicated in antifungal-induced stress. Additional modulators of cell wall integrity include the casein kinase 1 (CK1) family members, Yck2 and Hrr25, with the latter kinase also serving as a regulator of cell membrane homoeostasis. Downstream of both Hrr25 and the PKC-MAPK cascade is the SBF transcription factor (Swi4/Swi6), which is an important mediator of both cell wall and membrane integrity. There are also several additional elusive factors that remain to be identified.
Another critical mechanism of azole resistance that relies on stress responses involves mutations in the Δ-5,6-desaturase gene, ERG3. Specifically, loss-of-function mutations in ERG3 lead to a block in the accumulation of the toxic sterol 14-α-methyl-3,6-diol, which would otherwise accumulate in response to azole-mediated Erg11 inhibition7. In C. albicans, erg3-dependent azole resistance is contingent on Hsp90, calcineurin, and Pkc175,78. While the role of ERG3 mutations in azole resistance has been less evident in C. glabrata, recent work demonstrated that mutations in ERG3 can be acquired upon echinocandin exposure in vitro and confer cross-resistance to fluconazole36. Although questions remain as to why and how mutations in ERG3 emerged upon treatment with the cell-wall targeting echinocandins, this work contributes to a growing body of literature describing this phenomenon. In the related pathogen Candida parapsilosis, mutations in ERG3 have been reported in strains resistant to azoles and echinocandins80. Further, ERG3 mutations have also been identified in C. auris following echinocandin exposure42. More detailed analysis of the mechanism(s) underpinning erg3-mediated cross-resistance has the potential to illuminate the evolution of multidrug resistance in these emerging pathogens, which is important given that echinocandin treatment is now recommended as a first-line of defence for treating invasive candidiasis, and thus these reported multi-drug resistant phenotypes have important clinical implications.
In the context of echinocandins, cell wall salvage responses play integral roles in withstanding stress induced by the inhibition of glucan synthase. In C. albicans and A. fumigatus, one central mechanism involves the compensatory upregulation of chitin, an essential fungal cell wall polysaccharide81. In response to caspofungin, elevated levels of cell wall chitin can lead to attenuated antifungal activity at high concentrations, partially resorting growth through a phenomenon termed the paradoxical growth effect (or Eagle effect)81. In C. albicans, this compensatory chitin response to caspofungin depends on the core stress response regulators PKC, calcineurin, and the high-osmolarity glycerol (HOG) MAP kinase82. For A. fumigatus, the chitin biosynthetic response is controlled, at least in part, by calcineurin-mediated transcriptional upregulation of chitin synthases83.
Pkc1 has been extensively studied in the model yeast S. cerevisiae for its essential role in cell wall integrity signalling84. In brief, cell wall stress signals are triggered through cell surface sensors to the Rho1 GTPase, which in turn activates Pkc1 and a linear cascade of MAP kinases. Effectors downstream of the PKC-MAPK cascade include the terminal transcription factors Rlm1 and SBF (Swi4/Swi6)84. In S. cerevisiae, Rlm1 is the major regulator of cell wall integrity and its transcriptional output includes genes involved in cell wall biogenesis, while SBF is a cell cycle transcription factor that regulates the transcription of cell wall-related genes including FKS2 under conditions of cell wall stress84. As discussed previously, PKC also regulates membrane stress responses in C. albicans and this process is largely dependent on Rlm178. In addition to Pkc1, several other kinases have been recognized for mediating antifungal drug resistance. One such kinase is the target of rapamycin (TOR), which plays a central role in nutrient sensing and metabolism in eukaryotes. In C. albicans, hyperactivation of TOR can be used to bypass azole toxicity85. TOR mediates this effect by activating Hsp90 via modulation of casein kinase 2 (CK2) activity, leading to calcineurin stabilization85. In line with this, rapamycin blocks azole resistance acquired by loss-of-function of Erg3, a mode of resistance dependent upon Hsp9086. The natural product beauvericin, which potentiates azole activity via both inhibition of TOR and multidrug efflux, is effective against diverse fungal pathogens including C. albicans, C. neoformans, and A. fumigatus87. More recently, the casein kinase 1 (CK1) family members, Yck2 and Hrr25, were implicated in FKS1-mediated echinocandin resistance in C. albicans88,89. Hrr25 was also demonstrated to be required for azole tolerance in a clinical isolate89. Further mechanistic investigations determined that Hrr25 maintains cell wall homoeostasis by interacting with the SBF (Swi4/Swi6) transcription factor89. Given that SBF is a well-characterized downstream effector of the PKC-MAPK cascade, this highlights the potential crosstalk between Hrr25 and Pkc1 signalling in regulating drug-induced stress responses78,89, and thus would be an interesting avenue of future study.
Epigenetic regulation
There has been a growing appreciation for epigenetic factors mediating acquired antifungal drug resistance. One well-characterized chromatin-based modification involves the addition/removal of acetyl groups to/from the lysine residues of histone tails or other cellular proteins. For instance, the expression of lysine deacetylases HDA1 and RPD3 are upregulated in fluconazole-resistant C. albicans isolates, specifically in the early stages of resistance acquisition90. Furthermore, deacetylation is required for Hsp90 function, such that pharmacological or genetic inhibition of lysine deacetylases impedes the physical interaction between Hsp90 and calcineurin, thereby blocking the induction of key stress responses required for maintenance of Hsp90-dependent azole resistance91. Acetylation-dependent regulation of Hsp90 is also crucial for azole and echinocandin resistance in A. fumigatus, with the lysine 27 residue playing a vital role in this process92. Lysine acetyltransferases, such as Gcn5, also mediate antifungal tolerance and resistance in Candida species93,94,95. Notably, in C. glabrata, inhibition of Gcn5 minimized the emergence of PDR1 gain-of-function alleles in evolution experiments with fluconazole, suggesting the importance of histone modifications not only in the maintenance of azole resistance but also in the acquisition of azole resistance95.
Another significant post-translational modification in histone proteins involves the addition/removal of methyl groups by histone methyltransferases and demethylases. Despite the integral role of histone methylation on gene regulation, its impact on resistance to antifungals has only been very recently explored. In C. glabrata, disruption of RPH1, which encodes a putative histone demethylase, causes increased sensitivity to fluconazole and reduced basal expression of the canonical azole resistance-related genes PDR1 and CDR196. In addition, the histone H3K4 methyltransferase Set1 modulates responses to azoles as it is necessary for azole-induced expression of ergosterol biosynthesis genes, including ERG11 and ERG397.
Beyond histone modifications, chromatin remodelling complexes have also been implicated in antifungal resistance. In C. albicans, the SWI/SNF chromatin remodelling complex is a crucial coactivator of the fluconazole resistance-related transcription factor Mrr198. Deletion of SNF2, which encodes the SWI/SNF catalytic subunit, significantly reduces the upregulation of MDR1 expression and fluconazole resistance in MRR1 gain-of-function strains98. In C. glabrata, the SWI/SNF complex, along with the histone chaperone Rtt106, mediate azole resistance by driving the expression of the multidrug transporter genes, including CDR199. Notably, Rtt106 and some SWI/SNF subunits are fungal-specific and thus may serve as promising therapeutic targets to overcome antifungal resistance in this important pathogen99.
In addition to chromatin modifications, fungi also display RNA-based modes of antifungal resistance. In Mucor circinelloides (causative agent of mucormycosis), RNAi (RNA interference)-mediated epimutations can confer antifungal resistance via degradation of mRNAs of drug target genes100,101. Additional work showed the RNAi-dependent epimutation pathway is inhibited by the non-canonical RdRP-dependent Dicer-independent silencing pathway under non-stress conditions102. In a systemic mouse model of Mucor infection, epimutation-mediated drug resistance can be rapidly induced and reverted over the course of the infection, and thus represents a potential clinically-relevant factor affecting drug resistance103. Future work should determine whether epimutation-mediated drug resistance can be generalized to other fungal pathogens with functional RNAi machinery, including C. neoformans and A. fumigatus. It is noteworthy that the canonical RNAi pathway has been lost in C. albicans and other budding yeast species; however, these organisms possess noncanonical RNAi proteins104.
Given the ubiquitous nature of RNAi, there has been growing interest in RNA-based therapeutics for the treatment of fungal infections, particularly in the management of crop diseases105. While RNA-based strategies for combatting fungal infections in humans have received far less attention, it offers an alternative strategy to conventional drugs. While significant challenges with the delivery of antifungal RNAs include fungal cell permeability and susceptibility to degradation by RNases in the host environment, several promising approaches are underway in related systems, which may be applied to the treatment of fungal infections106. Advancing our understanding of RNAi systems in human fungal pathogens is poised to reveal suitable targets that may be exploited for RNA-based therapeutics to combat mycotic disease.
Concluding remarks
Our scarce antifungal arsenal, combined with the rising prevalence of drug-resistant fungal pathogens, represents a significant clinical challenge for the present and future. Beyond the overwhelming impact of fungi on humans, fungal infections also cause massive declines in crop production and wildlife viability, posing a growing threat to worldwide food security and ecosystem health107. Despite the challenges inherent to antifungal development, considerable progress has been made to expand our antifungal arsenal in recent years. Antifungal agents with distinct modes of action in development include olorofim (which blocks de novo pyrimidine synthesis), fosmanogepix (which blocks glycosylphosphatidylinositol (GPI) production), and ibrexafungerp (which inhibits glucan synthesis via a mechanism distinct of the echinocandins)108. Additionally, several agents within existing antifungal drug classes with improved pharmacokinetics and safety are currently in development, such as the echinocandin rezafungin, which possesses a prolonged half-life, and the tetrazole oteseconazole, which exhibits higher specificity for fungal CYP51, minimizing drug-drug interactions108.
The clinically-available armamentarium for antifungal treatment currently relies on targeting essential gene products or cellular processes in fungi. One promising alternative strategy to expand the antifungal target space and impede the evolution of resistance is the use of combination therapy (Fig. 3)109. Indeed, this approach has been proven efficacious for treating various infectious diseases such as malaria and AIDS/HIV109. Notably, recent work demonstrated that impairing efflux might be an effective strategy to restore azole sensitivity in the multidrug-resistant pathogens C. auris and C. glabrata110,111. In addition to resistance mechanisms, combination therapy can also involve the inhibition of critical regulatory hubs or modulators of cellular stress responses, such as the molecular chaperone Hsp90 and its client protein calcineurin (Fig. 3)112,113. While the conservation of such targets in humans poses challenges, structure-guided analyses can aid the design of fungal-specific inhibitors to minimize harmful effects on the host114,115. Beyond the optimization of existing drugs, high-throughput screening of compound libraries coupled with chemical genomic resources in fungal pathogens can facilitate the discovery of chemical matter to bolster the antifungal pipeline and enable the development of resistance-aversive therapies116. Continued efforts to advance our mechanistic understanding of antifungal resistance will undoubtedly support the development of therapeutic strategies to combat invasive fungal infections.
Compared with monotherapy, treatment with drug combinations can improve drug efficacy and overcome resistance. a Targeting resistance mechanisms to improve antifungal efficacy due to increased bioavailability against multidrug-resistant pathogens. For example, pharmacological inhibition of efflux pump Cdr1 with a bis-benzodioxolylindolinone (azoffluxin) increases intracellular fluconazole levels, improving fluconazole activity against the emerging pathogen C. auris. b Targeting stress response regulators to enhance antifungal efficacy and impede the emergence of drug resistance. For example, pharmacological inhibition of Hsp90 with geldanamycin abrogates stress responses required to survive antifungal-induced stress.
Data availability
The authors confirm that the data supporting the findings of this review are available within the article or are available from the corresponding author upon reasonable request.
References
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13–165rv13 (2012).
Pfaller, M. A. & Diekema, D. J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36, 1–53 (2010).
Rajasingham, R. et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet. Infect. Dis. 22, 1748–1755 (2022).
Anderson, T. M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014).
Roemer, T. & Krysan, D. J. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold. Spring. Harb. Perspect. Med. 4, a019703 (2014).
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N. & Cowen, L. E. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 19, 454–466 (2021).
Shapiro, R. S., Robbins, N. & Cowen, L. E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75, 213–267 (2011).
Pfaller, M. A., Diekema, D. J., Turnidge, J. D., Castanheira, M. & Jones, R. N. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open. Forum. Infect. Dis. 6, S79–S94 (2019).
Morio, F., Loge, C., Besse, B., Hennequin, C. & le Pape, P. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn. Microbiol. Infect. Dis. 66, 373–384 (2010).
Marichal, P. et al. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology (Reading). 145, 2701–2713 (1999).
Hargrove, T. Y. et al. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 292, 6728–6743 (2017).
Chowdhary, A. et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 73, 891–899 (2018).
Healey, K. R. et al. Limited ERG11 mutations identified in isolates of Candida auris directly contribute to reduced azole susceptibility. Antimicrob. Agents. Chemother. 62, e01427–18 (2018).
Rybak, J. M. et al. Delineation of the direct contribution of Candida auris ERG11 mutations to clinical triazole resistance. Microbiol. Spectr. 9, e0158521 (2021).
Li, J. et al. Novel ERG11 and TAC1b mutations associated with azole resistance in Candida auris. Antimicrob. Agents. Chemother. 65, e02663–20 (2021).
Rodero, L. et al. G484S amino acid substitution in lanosterol 14-alpha demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob. Agents. Chemother. 47, 3653–3656 (2003).
Handelman, M. et al. Point mutation or overexpression of Aspergillus fumigatus cyp51B, encoding lanosterol 14α-sterol demethylase, leads to triazole resistance. Antimicrob. Agents. Chemother. 65, e0125221 (2021).
Howard, S. J. & Arendrup, M. C. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49, S90–S95 (2011).
Sewell, T. R. et al. Nonrandom distribution of azole resistance across the global population of Aspergillus fumigatus. mBio. 10, e00392–19 (2019).
Schoustra, S. E. et al. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, the Netherlands. Emerg. Infect. Dis. 25, 1347–1353 (2019).
Hurst, S. F., Berkow, E. L., Stevenson, K. L., Litvintseva, A. P. & Lockhart, S. R. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J. Antimicrob. Chemother. 72, 2443–2446 (2017).
Alvarez-Moreno, C. et al. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci. Rep. 7, 45631 (2017).
Etienne, K. A. et al. Genomic diversity of azole-resistant Aspergillus fumigatus in the United States. mBio. 12, e0180321 (2021).
Silver, P. M., Oliver, B. G. & White, T. C. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell. 3, 1391–1397 (2004).
Yang, H. et al. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 6, 6129 (2015).
Tan, L. et al. Structural basis for activation of fungal sterol receptor Upc2 and azole resistance. Nat. Chem. Biol. 18, 1253–1262 (2022).
Vu, B. G., Stamnes, M. A., Li, Y., Rogers, P. D. & Moye-Rowley, W. S. The Candida glabrata Upc2A transcription factor is a global regulator of antifungal drug resistance pathways. PLoS. Genet. 17, e1009582 (2021).
Willger, S. D. et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS. Pathog. 4, e1000200 (2008).
Chang, Y. C., Bien, C. M., Lee, H., Espenshade, P. J. & Kwon-Chung, K. J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 64, 614–629 (2007).
Hagiwara, D. et al. A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. PLoS. Pathog. 13, e1006096 (2017).
Paul, S. et al. AtrR is an essential determinant of azole resistance in Aspergillus fumigatus. mBio. 10, e02563–18 (2019).
Du, W. et al. The C2H2 transcription factor SltA contributes to azole resistance by coregulating the expression of the drug target Erg11A and the drug efflux pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents. Chemother. 65, e01839–20 (2021).
Garcia-Effron, G., Lee, S., Park, S., Cleary, J. D. & Perlin, D. S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents. Chemother. 53, 3690–3699 (2009).
Park, S. et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents. Chemother. 49, 3264–3273 (2005).
Katiyar, S. K. et al. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob. Agents. Chemother. 56, 6304–6309 (2012).
Ksiezopolska, E. et al. Narrow mutational signatures drive acquisition of multidrug resistance in the fungal pathogen Candida glabrata. Curr. Biol. 31, 5314–5326.e10 (2021).
Kordalewska, M. et al. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrob. Agents. Chemother. 62, e00238–18 (2018).
Al-Obaid, I. et al. Fatal breakthrough candidemia in an immunocompromised patient in Kuwait due to Candida auris exhibiting reduced susceptibility to echinocandins and carrying a novel mutation in hotspot-1 of FKS1. J. Fungi (Basel). 8, 267 (2022).
Sharma, D. et al. Impact of FKS1 genotype on echinocandin in vitro susceptibility in Candida auris and in vivo response in a murine model of infection. Antimicrob. Agents. Chemother. 66, e0165221 (2022).
Asadzadeh, M. et al. Molecular characterisation of Candida auris isolates from immunocompromised patients in a tertiary-care hospital in Kuwait reveals a novel mutation in FKS1 conferring reduced susceptibility to echinocandins. Mycoses. 65, 331–343 (2022).
Rhodes, J. et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes. Infect. 7, 43 (2018).
Carolus, H. et al. Genome-wide analysis of experimentally evolved Candida auris reveals multiple novel mechanisms of multidrug resistance. mBio. 12, e03333–20 (2021).
Hu, X. et al. Structural and mechanistic insights into fungal β-1,3-glucan synthase. FKS1. Nature. 616, 190–198 (2023).
Lewis, R. E., Liao, G., Hou, J., Prince, R. A. & Kontoyiannis, D. P. Comparative in vivo dose-dependent activity of caspofungin and anidulafungin against echinocandin-susceptible and -resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 66, 1324–1331 (2011).
Vincent, B. M., Lancaster, A. K., Scherz-Shouval, R., Whitesell, L. & Lindquist, S. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS. Biol. 11, e1001692 (2013).
Vandeputte, P. et al. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents. Chemother. 52, 3701–3709 (2008).
Rybak, J. M. et al. In vivo emergence of high-level resistance during treatment reveals the first identified mechanism of amphotericin B resistance in Candida auris. Clin. Microbiol. Infect. 28, 838–843 (2022).
Kelly, S. L. et al. Resistance to amphotericin B associated with defective sterol delta 8–>7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS. Microbiol. Lett. 122, 39–42 (1994).
Cannon, R. D. et al. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22, 291–321 (2009).
Sanglard, D. et al. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents. Chemother. 39, 2378–2386 (1995).
Sanglard, D., Ischer, F., Monod, M. & Bille, J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology (Reading). 143, 405–416 (1997).
Coste, A. et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 172, 2139–2156 (2006).
Dunkel, N., Blaß, J., Rogers, P. D. & Morschhäuser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69, 827–840 (2008).
Rybak, J. M. et al. Mutations in TAC1B: a novel genetic determinant of clinical fluconazole resistance in Candida auris. mBio. 11, e00365–20 (2020).
Li, J., Coste, A. T., Bachmann, D., Sanglard, D. & Lamoth, F. Deciphering the Mrr1/Mdr1 pathway in azole resistance of Candida auris. Antimicrob. Agents. Chemother. 66, e0006722 (2022).
Rybak, J. M., Cuomo, C. A. & David Rogers, P. The molecular and genetic basis of antifungal resistance in the emerging fungal pathogen Candida auris. Curr. Opin. Microbiol. 70, 102208 (2022).
Tsai, H.-F., Krol, A. A., Sarti, K. E. & Bennett, J. E. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob. Agents. Chemother. 50, 1384–1392 (2006).
Vermitsky, J.-P. & Edlind, T. D. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents. Chemother. 48, 3773–3781 (2004).
Vu, B. G., Thomas, G. H. & Moye-Rowley, W. S. Evidence that ergosterol biosynthesis modulates activity of the Pdr1 transcription factor in Candida glabrata. mBio. 10, e00934–19 (2019).
Slaven, J. W. et al. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal. Genet. Biol. 36, 199–206 (2002).
Posteraro, B. et al. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 47, 357–371 (2003).
Selmecki, A., Forche, A. & Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 313, 367–370 (2006).
Selmecki, A., Gerami-Nejad, M., Paulson, C., Forche, A. & Berman, J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 68, 624–641 (2008).
Bing, J. et al. Experimental evolution identifies adaptive aneuploidy as a mechanism of fluconazole resistance in Candida auris. Antimicrob. Agents. Chemother. 65, e01466-20 (2020).
Coste, A. et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell. 6, 1889–1904 (2007).
Yang, F. et al. Aneuploidy enables cross-adaptation to unrelated drugs. Mol. Biol. Evol. 36, 1768–1782 (2019).
Yang, F. et al. Tunicamycin potentiates antifungal drug tolerance via aneuploidy in Candida albicans. mBio. 12, e0227221 (2021).
Todd, R. T. & Selmecki, A. Expandable and reversible copy number amplification drives rapid adaptation to antifungal drugs. Elife. 9, e58349 (2020).
Todd, R. T. et al. Antifungal drug concentration impacts the spectrum of adaptive mutations in Candida albicans. Mol. Biol. Evol. 40, msad009 (2023).
Poláková, S. et al. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc. Natl. Acad. Sci. 106, 2688–2693 (2009).
Sionov, E., Lee, H., Chang, Y. C. & Kwon-Chung, K. J. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS. Pathog. 6, e1000848 (2010).
Morogovsky, A., Handelman, M., Abou Kandil, A., Shadkchan, Y. & Osherov, N. Horizontal gene transfer of triazole resistance in Aspergillus fumigatus. Microbiol. Spectr. 10, e0111222 (2022).
Fitzpatrick, D. A. Horizontal gene transfer in fungi. FEMS. Microbiol. Lett. 329, 1–8 (2012).
Cordeiro, R. A. et al. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology (Reading). 162, 309–317 (2016).
Cowen, L. E. & Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 309, 2185–2189 (2005).
Singh, S. D. et al. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS. Pathog. 5, e1000532 (2009).
Caplan, T. et al. Functional genomic screening reveals core modulators of echinocandin stress responses in Candida albicans. Cell. Rep. 23, 2292–2298 (2018).
LaFayette, S. L. et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, Calcineurin, and Hsp90. PLoS. Pathog. 6, e1001069 (2010).
Fu, C. et al. Genetic analysis of Hsp90 function in Cryptococcus neoformans highlights key roles in stress tolerance and virulence. Genetics. 220, iyab164 (2022).
Papp, C. et al. Triazole evolution of Candida parapsilosis results in cross-resistance to other antifungal drugs, influences stress responses, and alters virulence in an antifungal drug-dependent manner. mSphere. 5, e00821–20 (2020).
Walker, L. A., Gow, N. A. R. & Munro, C. A. Fungal echinocandin resistance. Fungal. Genet. Biol. 47, 117–126 (2010).
Munro, C. A. et al. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63, 1399–1413 (2007).
Fortwendel, J. R. et al. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents. Chemother. 54, 1555–1563 (2010).
Levin, D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69, 262–291 (2005).
Khandelwal, N. K. et al. Azole resistance in a Candida albicans mutant lacking the ABC transporter CDR6/ROA1 depends on TOR signaling. J. Biol. Chem. 293, 412–432 (2018).
Robbins, N., Collins, C., Morhayim, J. & Cowen, L. E. Metabolic control of antifungal drug resistance. Fungal. Genet. Biol. 47, 81–93 (2010).
Shekhar-Guturja, T. et al. Dual action antifungal small molecule modulates multidrug efflux and TOR signaling. Nat. Chem. Biol. 12, 867–875 (2016).
Caplan, T. et al. Overcoming fungal echinocandin resistance through inhibition of the non-essential stress kinase Yck2. Cell. Chem. Biol. 27, 269–282.e5 (2020).
Lee, Y., Liston, S. D., Lee, D., Robbins, N. & Cowen, L. E. Functional analysis of the Candida albicans kinome reveals Hrr25 as a regulator of antifungal susceptibility. iScience. 25, 104432 (2022).
Li, X. et al. The Rpd3/Hda1 family of histone deacetylases regulates azole resistance in Candida albicans. J. Antimicrob. Chemother. 70, 1993–2003 (2015).
Robbins, N., Leach, M. D. & Cowen, L. E. Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance. Cell. Rep. 2, 878–888 (2012).
Lamoth, F. et al. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents. Chemother. 58, 1889–1896 (2014).
Yu, S. et al. Histone acetylation regulator Gcn5 mediates drug resistance and virulence of Candida glabrata. Microbiol. Spectr. 10, e0096322 (2022).
Shivarathri, R. et al. The fungal histone acetyl transferase Gcn5 controls virulence of the human pathogen Candida albicans through multiple pathways. Sci. Rep. 9, 9445 (2019).
Usher, J. & Haynes, K. Attenuating the emergence of anti-fungal drug resistance by harnessing synthetic lethal interactions in a model organism. PLoS. Genet. 15, e1008259 (2019).
Moirangthem, R., Kumar, K. & Kaur, R. Two functionally redundant FK506-binding proteins regulate multidrug resistance gene expression and govern azole antifungal resistance. Antimicrob. Agents. Chemother. 65, e02415-20 (2021).
Baker, K. M. et al. The Set1 histone H3K4 methyltransferase contributes to azole susceptibility in a species-specific manner by differentially altering the expression of drug efflux pumps and the ergosterol gene pathway. Antimicrob. Agents. Chemother. 66, e0225021 (2022).
Liu, Z. & Myers, L. C. Candida albicans Swi/Snf and mediator complexes differentially regulate Mrr1-Induced MDR1 expression and fluconazole resistance. Antimicrob. Agents. Chemother. 61, e01344-17 (2017).
Nikolov, V. N., Malavia, D. & Kubota, T. SWI/SNF and the histone chaperone Rtt106 drive expression of the pleiotropic drug resistance network genes. Nat. Commun. 13, 1968 (2022).
Chang, Z., Billmyre, R. B., Lee, S. C. & Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS. Genet. 15, e1007957 (2019).
Calo, S. et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature. 513, 555–558 (2014).
Calo, S. et al. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS. Genet. 13, e1006686 (2017).
Chang, Z. & Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. mBio. 10, e02579–19 (2019).
Drinnenberg, I. A. et al. RNAi in budding yeast. Science. 326, 544–550 (2009).
Cai, Q., He, B., Kogel, K.-H. & Jin, H. Cross-kingdom RNA trafficking and environmental RNAi—nature’s blueprint for modern crop protection strategies. Curr. Opin. Microbiol. 46, 58–64 (2018).
Bruch, A., Kelani, A. A. & Blango, M. G. RNA-based therapeutics to treat human fungal infections. Trends. Microbiol. 30, 411–420 (2022).
Fisher, M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 484, 186–194 (2012).
Gow, N. A. R. et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 13, 5352 (2022).
Robbins, N., Caplan, T. & Cowen, L. E. Molecular evolution of antifungal drug resistance. Annu. Rev. Microbiol. 71, 753–775 (2017).
Iyer, K. R. et al. An oxindole efflux inhibitor potentiates azoles and impairs virulence in the fungal pathogen Candida auris. Nat. Commun. 11, 6429 (2020).
Nishikawa, J. L. et al. Inhibiting fungal multidrug resistance by disrupting an activator-mediator interaction. Nature. 530, 485–489 (2016).
Cowen, L. E. et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. 106, 2818–2823 (2009).
Steinbach, W. J., Reedy, J. L., Cramer, R. A., Perfect, J. R. & Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5, 418–430 (2007).
Whitesell, L. et al. Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus. Nat. Commun. 10, 402 (2019).
Juvvadi, P. R. et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat. Commun. 10, 4275 (2019).
Robbins, N. & Cowen, L. E. Antifungal discovery. Curr. Opin. Microbiol. 69, 102198 (2022).
Acknowledgements
We thank all members of the Cowen lab for helpful discussions. L.E.C. is supported by the CIHR Foundation grant (FDN-154288), as well as by National Institutes of Health NIAID R01 grants (R01AI162789 and R01AI1654661). L.E.C. is a Canada Research Chair (Tier 1) in Microbial Genomics & Infectious Disease and co-Director of the CIFAR Fungal Kingdom: Threats & Opportunities program.
Author information
Authors and Affiliations
Contributions
Y.L. wrote the article. N.R. and L.E.C. reviewed/edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
L.E.C. is a co-founder and shareholder in Bright Angel Therapeutics, a platform company for development of novel antifungal therapeutics. L.E.C. is a Science Advisor for Kapoose Creek, a company that harnesses the therapeutic potential of fungi. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, Y., Robbins, N. & Cowen, L.E. Molecular mechanisms governing antifungal drug resistance. npj Antimicrob Resist 1, 5 (2023). https://doi.org/10.1038/s44259-023-00007-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44259-023-00007-2
This article is cited by
-
Molecular basis of the inositol deacylase PGAP1 involved in quality control of GPI-AP biogenesis
Nature Communications (2024)
-
Recent Advances and Future Perspectives in Mitigating Invasive Antifungal-Resistant Pathogen Aspergillus fumigatus in Africa
Current Treatment Options in Infectious Diseases (2023)