Abstract
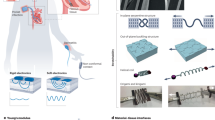
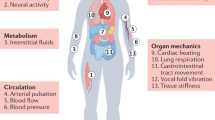
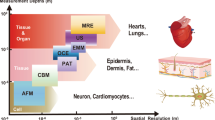
Soft bioelectronic devices can be interfaced with anatomically curved organs, such as the heart, brain and skin, to provide continuous analysis of physiological information. However, body movements and physiological activities may induce motion artefacts, which can adversely affect signal accuracy and stability. Importantly, motion artefact management is key to promoting the clinical translation of soft bioelectronics to ensure that soft bioelectronic devices can selectively detect target biological signals with high accuracy. In this Review, we discuss how body activities can affect the soft bioelectronic–tissue interface and result in motion artefact signals, including interface impedance instability motion artefacts, biopotential motion artefacts and mechanical motion artefacts. We then investigate different motion artefact management strategies, including materials engineering, device and circuit design, and algorithmic intervention, to reduce the contribution of motion artefacts to signal acquisition, processing and interpretation.
Key points
-
Soft bioelectronic systems for the monitoring of human health rely on a stable and conformal bioelectronic–tissue interface.
-
Motion artefacts can occur due to body movements and physiological activities, and can negatively affect signal detection and interpretation in bioelectronic measurements.
-
Motion artefact management is crucial to the clinical translation of soft bioelectronics to ensure measurement with high accuracy.
-
Materials usage, device design, bioelectronic–tissue adhesion, sensor and circuit designs, and algorithmic intervention are effective motion artefact management strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Williams, G. J. et al. Wearable technology and the cardiovascular system: the future of patient assessment. Lancet Digit. Health 5, e467–e476 (2023).
Sunwoo, S.-H. et al. Soft bioelectronics for the management of cardiovascular diseases. Nat. Rev. Bioeng. 2, 8–24 (2024).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022). This article summarizes biochemical sensor platforms that can be integrated with human skin for wearable health-care monitoring.
Cho, K. W. et al. Soft bioelectronics based on nanomaterials. Chem. Rev. 122, 5068–5143 (2022).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Haque, A., Milstein, A. & Fei-Fei, L. Illuminating the dark spaces of healthcare with ambient intelligence. Nature 585, 193–202 (2020).
Chen, G. et al. Electronic textiles for wearable point-of-care systems. Chem. Rev. 122, 3259–3291 (2022).
Libanori, A., Chen, G., Zhao, X., Zhou, Y. & Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 5, 142–156 (2022).
Meng, K. et al. A wireless textile-based sensor system for self-powered personalized health care. Matter 2, 896–907 (2020).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Gao, Q. et al. Highly stretchable sensors for wearable biomedical applications. J. Mater. Sci. 54, 5187–5223 (2019).
Wang, C., Yokota, T. & Someya, T. Natural biopolymer-based biocompatible conductors for stretchable bioelectronics. Chem. Rev. 121, 2109–2146 (2021).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Li, Y. et al. Achieving tissue-level softness on stretchable electronics through a generalizable soft interlayer design. Nat. Commun. 14, 4488 (2023).
Liang, H., Lin, Z. & Yin, F. Removal of ECG contamination from diaphragmatic EMG by nonlinear filtering. Nonlinear Anal. Theory Methods Appl. 63, 745–753 (2005).
Wang, G., Teng, C., Li, K., Zhang, Z. & Yan, X. The removal of EOG artifacts from EEG signals using independent component analysis and multivariate empirical mode decomposition. IEEE J. Biomed. Health Inform. 20, 1301–1308 (2016).
Lai, C. Q. et al. Artifacts and noise removal for electroencephalogram (EEG): a literature review. In 2018 IEEE Symposium on Computer Applications & Industrial Electronics (ISCAIE) 326–332 (IEEE, 2018).
Webster, J. G. Reducing motion artifacts and interference in biopotential recording. IEEE Trans. Biomed. Eng. 31, 823–826 (1984).
Rodeheaver, N. et al. Strain‐isolating materials and interfacial physics for soft wearable bioelectronics and wireless, motion artifact-controlled health monitoring. Adv. Funct. Mater. 31, 2104070 (2021).
Nan, K. et al. Mucosa-interfacing electronics. Nat. Rev. Mater. 7, 908–925 (2022).
Choi, H. et al. Adhesive bioelectronics for sutureless epicardial interfacing. Nat. Electron. 6, 779–789 (2023).
Boehler, C., Carli, S., Fadiga, L., Stieglitz, T. & Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020).
Jiang, Y. & Tian, B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 3, 473–490 (2018). This article discusses fundamental semiconductor physics and operation principles with a focus on biointerface establishment under physiological conditions.
Driscoll, N. et al. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Transl. Med. 13, eabf8629 (2021).
Strakosas, X. et al. Metabolite-induced in vivo fabrication of substrate-free organic bioelectronics. Science 379, 795–802 (2023).
Kim, J., Ghaffari, R. & Kim, D.-H. The quest for miniaturized soft bioelectronic devices. Nat. Biomed. Eng. 1, 0049 (2017).
Xiao, X., Zhou, Y., Yin, J., Zhao, X. & Chen, J. In-ear electrophysichochemical sensing. Nat. Biomed. Eng. 7, 1207–1209 (2023).
Tang, X., Shen, H., Zhao, S., Li, N. & Liu, J. Flexible brain-computer interfaces. Nat. Electron. 6, 109–118 (2023).
Lee, S. et al. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 14, 156–160 (2019).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Liu, Z. et al. High-adhesion stretchable electrodes based on nanopile interlocking. Adv. Mater. 29, 2 (2017).
Qi, D. et al. Highly stretchable, compliant, polymeric microelectrode arrays for in vivo electrophysiological interfacing. Adv. Mater. 29, 40 (2017).
Zhao, Y. et al. Soft strain-insensitive bioelectronics featuring brittle materials. Science 378, 1222–1227 (2022).
Gablech, I. & Głowacki, E. D. State‐of-the-art electronic materials for thin films in bioelectronics. Adv. Electron. Mater. 9, 2300258 (2023).
He, H. et al. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 14, 759 (2023).
Wang, Y. et al. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 51, 3759–3793 (2022).
Yuk, H., Wu, J. & Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 7, 935–952 (2022).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2018).
Alizadeh-Meghrazi, M. et al. Evaluation of dry textile electrodes for long-term electrocardiographic monitoring. Biomed. Eng. Online 20, 68 (2021).
Buxi, D. et al. Correlation between electrode-tissue impedance and motion artifact in biopotential recordings. IEEE Sens. J. 12, 3373–3383 (2012).
Murphy, B. B. et al. Time evolution of the skin–electrode interface impedance under different skin treatments. Sensors 21, 5210 (2021).
Neuman, M. In: Medical Instrumentation: Application and Design 4th edn (ed Webster, J. G.) 189–240 (Wiley, 1998).
Reilly, R. B. & Lee, T. C. Electrograms (ECG, EEG, EMG, EOG). Technol. Health Care 18, 443–458 (2010).
Teplan, M. Fundamental of EEG measurement. Meas. Sci. Rev. 2, 1–11 (2002).
Elul, R. The genesis of the EEG. Int. Rev. Neurobiol. 15, 227–272 (1972).
Woestenburg, J. C., Verbaten, M. N. & Slangen, J. L. The removal of the eye-movement artifact from the EEG by regression analysis in the frequency domain. Biol. Psychol. 16, 127–147 (1983).
Kher, R. Signal processing techniques for removing noise from ECG signals. J. Biomed. Eng. Res. 3, 212573348 (2019).
Mithun, P., Pandey, P. C., Sebastian, T., Mishra, P. & Pandey, V. K. A wavelet based technique for suppression of EMG noise and motion artifact in ambulatory ECG. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 7087–7090 (2011).
Marouf, M., Saranovac, L. & Vukomanovic, G. Algorithm for EMG noise level approximation in ECG signals. Biomed. Signal Process. Control 34, 158–165 (2017).
Meng, K. et al. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 34, 2109357 (2022).
Su, Y. et al. Self-powered respiration monitoring enabled by a triboelectric nanogenerator. Adv. Mater. 33, 2101262 (2021).
Zhang, S. et al. Leveraging triboelectric nanogenerators for bioengineering. Matter 4, 845–887 (2021).
Jeong, H. et al. Differential cardiopulmonary monitoring system for artifact-canceled physiological tracking of athletes, workers, and COVID-19 patients. Sci. Adv. 7, eabg3092 (2021). This article reports a circuit compensation design to accurately measure various physiological and movement indicators while mitigating motion artefacts during human activities.
Kireev, D. et al. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 17, 864–870 (2022).
Sel, K., Osman, D. & Jafari, R. Non-invasive cardiac and respiratory activity assessment from various human body locations using bioimpedance. IEEE Open J. Eng. Med. Biol. 2, 210–217 (2021).
Kang, J. et al. Tough-interface-enabled stretchable electronics using non-stretchable polymer semiconductors and conductors. Nat. Nanotechnol. 17, 1265–1271 (2022).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Afanasenkau, D. et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 4, 1010–1022 (2020).
Bootsma, K. et al. 3D printing of an interpenetrating network hydrogel material with tunable viscoelastic properties. J. Mech. Behav. Biomed. Mater. 70, 84–94 (2017).
Li, G. et al. Highly conducting and stretchable double-network hydrogel for soft bioelectronics. Adv. Mater. 34, e2200261 (2022).
Yan, Z. et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 375, 852–859 (2022). This article describes an adaptable and breathable electronic membrane with consistent bioelectronic–tissue adhesion for interface impedance instability motion artefact management.
Song, K.-I. et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 11, 4195 (2020).
Seo, H. et al. Durable and fatigue-resistant soft peripheral neuroprosthetics for in vivo bidirectional signaling. Adv. Mater. 33, e2007346 (2021).
Liu, Y. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 38, 1031–1036 (2020).
Tringides, C. M. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021).
Koo, J. H., Song, J.-K., Kim, D.-H. & Son, D. Soft implantable bioelectronics. ACS Mater. Lett. 3, 1528–1540 (2021).
Yan, Z. et al. Thermal release transfer printing for stretchable conformal bioelectronics. Adv. Sci. 4, 1700251 (2017).
Xu, L. et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 5, 3329 (2014).
Yu, Y., Sanchez, D. & Lu, N. Work of adhesion/separation between soft elastomers of different mixing ratios. J. Mater. Res. 30, 2702–2712 (2015).
Choi, Y. S. et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 11, 5990 (2020).
Kang, J. et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 30, e1706846 (2018).
Lu, B. et al. Pure PEDOT:PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Liu, N. et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 3, e1700159 (2017).
Dickey, M. D. Stretchable and soft electronics using liquid metals. Adv. Mater. 29, 1606425 (2017).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Li, Y. et al. Ultrasensitive and ultrastretchable electrically self-healing conductors. Proc. Natl Acad. Sci. USA 120, e2300953120 (2023).
Ohm, Y. et al. An electrically conductive silver-polyacrylamide-alginate hydrogel composite for soft electronics. Nat. Electron. 4, 185–192 (2021).
Xu, Y. et al. Porous liquid metal-elastomer composites with high leakage resistance and antimicrobial property for skin-interfaced bioelectronics. Sci. Adv. 9, eadf0575 (2023).
Zheng, L. et al. Conductance-stable liquid metal sheath-core microfibers for stretchy smart fabrics and self-powered sensing. Sci. Adv. 7, eabg4041 (2021).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Song, E., Li, J., Won, S. M., Bai, W. & Rogers, J. A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 19, 590–603 (2020).
Zhang, Y. et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 5, eaaw1066 (2019).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Peñuela, L. et al. Atomic force microscopy for biomechanical and structural analysis of human dermis: a complementary tool for medical diagnosis and therapy monitoring. Exp. Dermatol. 27, 150–155 (2018).
Choi, C., Lee, Y., Cho, K. W., Koo, J. H. & Kim, D.-H. Wearable and implantable soft bioelectronics using two-dimensional materials. Acc. Chem. Res. 52, 73–81 (2019).
Lee, Y. K. et al. Chemical sensing systems that utilize soft electronics on thin elastomeric substrates with open cellular designs. Adv. Funct. Mater. 27, 1605476 (2017).
Song, E. et al. Miniaturized electromechanical devices for the characterization of the biomechanics of deep tissue. Nat. Biomed. Eng. 5, 759–771 (2021).
Jeong, J. et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 25, 6839–6846 (2013). This article reports soft bioelectronics with a stretchable serpentine structure and thickness reduction engineering strategies for interface impedance instability motion artefact management.
Viventi, J. et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl. Med. 2, 24ra22 (2010).
Lee, S. et al. A strain-absorbing design for tissue-machine interfaces using a tunable adhesive gel. Nat. Commun. 5, 5898 (2014).
Kim, D.-H. et al. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl Acad. Sci. USA 105, 18675–18680 (2008).
Rogers, J. A., Someya, T. & Huang, Y. Materials and mechanics for stretchable electronics. Science 327, 1603–1607 (2010).
Silva, C. A. et al. Liquid metal based island-bridge architectures for all printed stretchable electrochemical devices. Adv. Funct. Mater. 30, 2002041 (2020).
Wang, W. et al. Strain-insensitive intrinsically stretchable transistors and circuits. Nat. Electron. 4, 143–150 (2021).
Zhang, Y., Huang, Y. & Rogers, J. A. Mechanics of stretchable batteries and supercapacitors. Curr. Opin. Solid State Mater. Sci. 19, 190–199 (2015).
Kim, D.-H. et al. Stretchable and foldable silicon integrated circuits. Science 320, 507–511 (2008).
Ma, Y. et al. Design of strain-limiting substrate materials for stretchable and flexible electronics. Adv. Funct. Mater. 26, 5345–5351 (2016).
Wang, M. et al. Ultrastretchable MXene microsupercapacitors. Small 19, e2300386 (2023).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Yeo, W. et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 25, 2773–2778 (2013).
Zhang, Y. et al. Mechanics of ultra-stretchable self-similar serpentine interconnects. Acta Mater. 61, 7816–7827 (2013).
Xu, S. et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 4, 1543 (2013).
Norton, J. J. S. et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proc. Natl Acad. Sci. USA 112, 3920–3925 (2015).
Xu, L. et al. Materials and fractal designs for 3D multifunctional integumentary membranes with capabilities in cardiac electrotherapy. Adv. Mater. 27, 1731–1737 (2015).
Blees, M. K. et al. Graphene kirigami. Nature 524, 204–207 (2015).
Meng, K. et al. Kirigami-inspired pressure sensors for wearable dynamic cardiovascular monitoring. Adv. Mater. 34, 2202478 (2022). This article discusses a kirigami-inspired anisotropic response sensor design strategy for mechanical motion artefact management.
Brooks, A. K., Chakravarty, S., Ali, M. & Yadavalli, V. K. Kirigami‐inspired biodesign for applications in healthcare. Adv. Mater. 34, e2109550 (2022).
Luo, Y. et al. Learning human-environment interactions using conformal tactile textiles. Nat. Electron. 4, 193–201 (2021).
Chen, G., Fang, Y., Zhao, X., Tat, T. & Chen, J. Textiles for learning tactile interactions. Nat. Electron. 4, 175–176 (2021).
Chen, G., Li, Y., Bick, M. & Chen, J. Smart textiles for electricity generation. Chem. Rev. 120, 3668–3720 (2020).
Fang, Y., Chen, G., Bick, M. & Chen, J. Smart textiles for personalized thermoregulation. Chem. Soc. Rev. 50, 9357–9374 (2021).
Yin, J. et al. Smart textiles for self-powered biomonitoring. Med-X 1, 3 (2023).
Creton, C. Pressure-sensitive adhesives: an introductory course. MRS Bull. 28, 434–439 (2003).
Chung, J. Y. & Chaudhury, M. K. Soft and hard adhesion. J. Adhes. 81, 1119–1145 (2005).
Cong, Y. & Fu, J. Hydrogel-tissue interface interactions for implantable flexible bioelectronics. Langmuir 38, 11503–11513 (2022).
Bai, S. et al. Healable, transparent, room-temperature electronic sensors based on carbon nanotube network-coated polyelectrolyte multilayers. Small 11, 5807–5813 (2015).
Sun, T. L. et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 12, 932–937 (2013).
Long, T., Li, Y., Fang, X. & Sun, J. Salt‐mediated polyampholyte hydrogels with high mechanical strength, excellent self-healing property, and satisfactory electrical conductivity. Adv. Funct. Mater. 28, 1804416 (2018).
Roy, C. K. et al. Self‐adjustable adhesion of polyampholyte hydrogels. Adv. Mater. 27, 7344–7348 (2015).
Pan, L. et al. A compliant ionic adhesive electrode with ultralow bioelectronic impedance. Adv. Mater. 32, 2003723 (2020).
Hou, Y. et al. Tuning water-resistant networks in mussel-inspired hydrogels for robust wet tissue and bioelectronic adhesion. ACS Nano 17, 2745–2760 (2023).
Kim, S. et al. Injection-on-skin granular adhesive for interactive human–machine interface. Adv. Mater. 35, e2307070 (2023).
Saiz-Poseu, J., Mancebo-Aracil, J., Nador, F., Busqué, F. & Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. Engl. 58, 696–714 (2019).
Han, L. et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 13, 1601916 (2017).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Yang, S. et al. Stretchable surface electromyography electrode array patch for tendon location and muscle injury prevention. Nat. Commun. 14, 6494 (2023).
Cadirov, N., Booth, J. A., Turner, K. L. & Israelachvili, J. N. Influence of humidity on grip and release adhesion mechanisms for gecko-inspired microfibrillar surfaces. ACS Appl. Mater. Interfaces 9, 14497–14505 (2017).
Ma, X. et al. Capillary-force-assisted clean-stamp transfer of two-dimensional materials. Nano Lett. 17, 6961–6967 (2017).
Cheng, S. & Robbins, M. O. Nanocapillary adhesion between parallel plates. Langmuir 32, 7788–7795 (2016).
Fan, J., Coninck, J. D., Wu, H. & Wang, F. Microscopic origin of capillary force balance at contact line. Phys. Rev. Lett. 124, 125502 (2020).
Liu, Y. et al. Capillary-force-induced cold welding in silver-nanowire-based flexible transparent electrodes. Nano Lett. 17, 1090–1096 (2017).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Pokrajac, L. et al. Nanotechnology for a sustainable future: addressing global challenges with the international network4sustainable nanotechnology. ACS Nano 15, 18608–18623 (2021).
Bae, W. G. et al. Enhanced skin adhesive patch with modulus-tunable composite micropillars. Adv. Healthc. Mater. 2, 109–113 (2013).
Kwak, M. K., Jeong, H. & Suh, K. Y. Rational design and enhanced biocompatibility of a dry adhesive medical skin patch. Adv. Mater. 23, 3949–3953 (2011).
Kim, T., Park, J., Sohn, J., Cho, D. & Jeon, S. Bioinspired, highly stretchable, and conductive dry adhesives based on 1D-2D hybrid carbon nanocomposites for all-in-one ECG electrodes. ACS Nano 10, 4770–4778 (2016).
Baik, S. et al. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546, 396–400 (2017).
Baik, S., Kim, J., Lee, H. J., Lee, T. H. & Pang, C. Highly adaptable and biocompatible octopus-like adhesive patches with meniscus-controlled unfoldable 3D microtips for underwater surface and hairy skin. Adv. Sci. 5, 1800100 (2018).
Choi, M. K. et al. Cephalopod-inspired miniaturized suction cups for smart medical skin. Adv. Healthc. Mater. 5, 80–87 (2016).
Chun, S. et al. Conductive and stretchable adhesive electronics with miniaturized octopus-like suckers against dry/wet skin for biosignal monitoring. Adv. Funct. Mater. 28, 1805224 (2018).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 8, 344ra86 (2016).
Sim, K. et al. Metal oxide semiconductor nanomembrane-based soft unnoticeable multifunctional electronics for wearable human-machine interfaces. Sci. Adv. 5, eaav9653 (2019).
Yang, Q., Hu, Z. & Rogers, J. A. Functional hydrogel interface materials for advanced bioelectronic devices. Acc. Mater. Res. 2, 1010–1023 (2021).
Kim, J., Oh, J., Park, Y., Kim, J. J. & Jeong, U. Soft conductive interfacing for bioelectrical uses: adhesion mechanisms and structural approaches. Macromolecules 56, 4431–4446 (2023).
Tian, G. et al. A nonswelling hydrogel with regenerable high wet tissue adhesion for bioelectronics. Adv. Mater. 35, 2212302 (2023).
Chen, X., Yuk, H., Wu, J., Nabzdyk, C. S. & Zhao, X. Instant tough bioadhesive with triggerable benign detachment. Proc. Natl Acad. Sci. USA 117, 15497–15503 (2020).
Zhan, Y., Halliday, D., Jiang, P., Liu, X. & Feng, J. Detecting time-dependent coherence between non-stationary electrophysiological signals-a combined statistical and time-frequency approach. J. Neurosci. Methods 156, 322–332 (2006).
Williams, W. J., Zaveri, H. P. & Sackellares, J. C. Time-frequency analysis of electrophysiology signals in epilepsy. IEEE Eng. Med. Biol. Mag. 14, 133–143 (1995).
Murthy, V. K., Grove, T. M., Harvey, G. A. & Haywood, L. J. Clinical usefulness of ECG frequency spectrum analysis. Proc. Annu. Symp. Comput. Appl. Med. Care 610–612 (1978).
Kostyunina, M. B. & Kulikov, M. A. Frequency characteristics of EEG spectra in the emotions. Neurosci. Behav. Physiol. 26, 340–343 (1996).
Al-Fahoum, A. S. & Al-Fraihat, A. A. Methods of EEG signal features extraction using linear analysis in frequency and time-frequency domains. ISRN Neurosci. 2014, 730218 (2014).
Komi, P. V. & Tesch, P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 42, 41–50 (1979).
Grosse, P., Cassidy, M. J. & Brown, P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: physiological principles and clinical applications. Clin. Neurophysiol. 113, 1523–1531 (2002).
Wang, Y. et al. Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale. Sci. Adv. 6, eabd0996 (2020). This article presents a signal compensation circuit using the differential method for biopotential and mechanical motion artefact management.
Sugiyama, M. et al. An ultraflexible organic differential amplifier for recording electrocardiograms. Nat. Electron. 2, 351–360 (2019).
Faisal, A. A., Selen, L. P. J. & Wolpert, D. M. Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303 (2008).
van Drongelen, W. Signal Processing for Neuroscientists 169–175 (Elsevier Science, 2018).
Lin, C.-F. & Zhu, J.-D. Hilbert-Huang transformation-based time-frequency analysis methods in biomedical signal applications. Proc. Inst. Mech. Eng. H 226, 208–216 (2012).
Challis, R. E. & Kitney, R. I. Biomedical signal processing, part 2: the frequency transforms and their inter-relationships. Med. Biol. Eng. Comput. 28, 509–524 (1990).
Gómez-Echavarría, A., Ugarte, J. P. & Tobón, C. The fractional Fourier transform as a biomedical signal and image processing tool: a review. Biocybern. Biomed. Eng. 40, 1081–1093 (2020).
Challis, R. E. & Kitney, R. I. The design of digital filters for biomedical signal processing part 3: the design of Butterworth and Chebychev filters. J. Biomed. Eng. 5, 91–102 (1983).
Sadasivan, P. K. & Dutt, D. N. Use of finite wordlength FIR digital filter structures with improved magnitude and phase characteristics for reduction of muscle noise in EEG signals. Med. Biol. Eng. Comput. 33, 306–312 (1995).
Correa, A. G., Laciar, E., Patiño, H. D. & Valentinuzzi, M. E. Artifact removal from EEG signals using adaptive filters in cascade. J. Phys. Conf. Ser. 90, 012081 (2007).
Krittanawong, C. et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat. Rev. Cardiol. 18, 75–91 (2021).
Li, Y. et al. Learning hand kinematics for Parkinson’s disease assessment using a multimodal sensor glove. Adv. Sci. 10, 2206982 (2023).
Xu, J. et al. Deep learning assisted ternary electrification layered triboelectric membrane sensor for self-powered home security. Nano Energy 113, 108524 (2023).
Dillen, A. et al. Deep learning for biosignal control: insights from basic to real-time methods with recommendations. J. Neural Eng. 19, 011003 (2022).
Sun, W., Su, Y., Wu, X. & Wu, X. A novel end-to-end 1D-ResCNN model to remove artifact from EEG signals. Neurocomputing 404, 108–121 (2020).
Xiong, D., Zhang, D., Zhao, X. & Zhao, Y. Deep learning for EMG-based human-machine interaction: a review. IEEE/CAA J. Autom. Sin. 8, 512–533 (2021).
Luca, C. J. D., Gilmore, L. D., Kuznetsov, M. & Roy, S. H. Filtering the surface EMG signal: movement artifact and baseline noise contamination. J. Biomech. 43, 1573–1579 (2010). This article reports on a signal processing strategy based on a Butterworth filtering algorithm in electrophysiological monitoring for mechanical motion artefact management.
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2018).
Flynn, C. D. et al. Biomolecular sensors for advanced physiological monitoring. Nat. Rev. Bioeng. 1, 560–575 (2023).
Chen, S. et al. Wearable flexible microfluidic sensing technologies. Nat. Rev. Bioeng. 1, 950–971 (2023).
Che, Z., Wan, X. & Xu, J. et al. Speaking without vocal folds using a machine-learning-assisted wearable sensing-actuation system. Nat. Commun. 15, 1873 (2024).
Zhou, Y. et al. A multimodal magnetoelastic artificial skin for underwater haptic sensing. Sci. Adv. 10, eadj8567 (2024).
Fang, Y. et al. Ambulatory cardiovascular monitoring via a machine-learning-assisted textile triboelectric sensor. Adv. Mater. 33, 2104178 (2021). This article presents a machine learning-assisted signal features extraction strategy for biopotential and mechanical motion artefact management.
Yi, Z. et al. Piezoelectric dynamics of arterial pulse for wearable continuous blood pressure monitoring. Adv. Mater. 34, 2110291 (2022).
Rowland, E. M., Bailey, E. L. & Weinberg, P. D. Estimating arterial cyclic strain from the spacing of endothelial nuclei. Exp. Mech. 61, 171–190 (2021).
Edin, B. B. & Johansson, N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J. Physiol. 487, 243–251 (1995).
Park, B. et al. Cuticular pad-inspired selective frequency damper for nearly dynamic noise-free bioelectronics. Science 376, 624–629 (2022).
Jeong, C. et al. Motion artifact-resilient zone for implantable sensors. Adv. Funct. Mater. 32, 2206461 (2022).
Rodeheaver, N., Kim, H., Herbert, R., Seo, H. & Yeo, W.-H. Breathable, wireless, thin-film wearable biopatch using noise-reduction mechanisms. ACS Appl. Electron. Mater. 4, 503–512 (2022).
Zhao, Y. et al. A wearable freestanding electrochemical sensing system. Sci. Adv. 6, eaaz0007 (2020).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Wang, W. et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 380, 735–742 (2023).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Dai, Y., Hu, H., Wang, M., Xu, J. & Wang, S. Stretchable transistors and functional circuits for human-integrated electronics. Nat. Electron. 4, 17–29 (2021).
Molina-Lopez, F. et al. Inkjet-printed stretchable and low voltage synaptic transistor array. Nat. Commun. 10, 2676 (2019).
Shim, H. et al. An elastic and reconfigurable synaptic transistor based on a stretchable bilayer semiconductor. Nat. Electron. 5, 660–671 (2022).
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Liu, K., Ouyang, B., Guo, X., Guo, Y. & Liu, Y. Advances in flexible organic field-effect transistors and their applications for flexible electronics. NPJ Flex. Electron. 6, 1 (2022).
Uemura, T. & Sekitani, T. Ultraflexible Biosignal Amplifier Based on Organic Thin-film Transistors. 2019 Compound Semiconductor Week (CSW). (IEEE, 2019).
Krishnan, S. & Athavale, Y. Trends in biomedical signal feature extraction. Biomed. Signal Process. Control 43, 41–63 (2018).
Lee, K. et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 4, 148–158 (2020).
Ni, X. et al. Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc. Natl Acad. Sci. USA 118, e2026610118 (2021).
Franklin, D. et al. Synchronized wearables for the detection of haemodynamic states via electrocardiography and multispectral photoplethysmography. Nat. Biomed. Eng. 7, 1229–1241 (2023).
Liu, J. et al. PCA-based multi-wavelength photoplethysmography algorithm for cuffless blood pressure measurement on elderly subjects. IEEE J. Biomed. Health Inform. 25, 663–673 (2020).
Shen, C. P. et al. Convolution neural network algorithm for shockable arrhythmia classification within a digitally connected automated external defibrillator. J. Am. Heart Assoc. 12, e026974 (2023).
Li, S., Lu, D., Pet, M., Rogers, J. A. & Huang, Y. An analytical model for sensing microvascular blood flow in flaps and organ grafts. J. Mech. Phys. Solids 170, 105119 (2023).
Webb, R. C. et al. Epidermal devices for noninvasive, precise, and continuous mapping of macrovascular and microvascular blood flow. Sci. Adv. 1, e1500701 (2015).
Lee, S. et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 11, 472–478 (2016).
Song, J.-K. et al. Stretchable colour-sensitive quantum dot nanocomposites for shape-tunable multiplexed phototransistor arrays. Nat. Nanotechnol. 17, 849–856 (2022).
Kim, S. H. et al. A bioinspired stretchable sensory-neuromorphic system. Adv. Mater. 33, e2104690 (2021).
Shi, X. et al. Large-area display textiles integrated with functional systems. Nature 591, 240–245 (2021).
He, J. et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 597, 57–63 (2021).
Ouyang, W. et al. A wireless and battery-less implant for multimodal closed-loop neuromodulation in small animals. Nat. Biomed. Eng. 7, 1252–1269 (2023).
Jin, S. et al. Injectable tissue prosthesis for instantaneous closed-loop rehabilitation. Nature 623, 58–65 (2023).
Luan, L. et al. Recent advances in electrical neural interface engineering: minimal invasiveness, longevity, and scalability. Neuron 108, 302–321 (2020).
Harrison, R. R. & Charles, C. A low-power low-noise CMOS amplifier for neural recording applications. IEEE J. Solid State Circuits 38, 958 (2003).
Ershad, F. et al. Ultra-conformal drawn-on-skin electronics for multifunctional motion artifact-free sensing and point-of-care treatment. Nat. Commun. 11, 3823 (2020).
Zhang, Y. et al. Printing, folding and assembly methods for forming 3D mesostructures in advanced materials. Nat. Rev. Mater. 2, 17019 (2017).
Liu, S. et al. Conformability of flexible sheets on spherical surfaces. Sci. Adv. 9, eadf2709 (2023).
Choi, C. et al. Human eye-inspired soft optoelectronic device using high-density MoS2-graphene curved image sensor array. Nat. Commun. 8, 1664 (2017).
Khan, Y. et al. A new frontier of printed electronics: flexible hybrid electronics. Adv. Mater. 32, e1905279 (2020).
Xu, Y. et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 7, 1307–1320 (2023).
Balakrishnan, G., Song, J., Mou, C. & Bettinger, C. J. Recent progress in materials chemistry to advance flexible bioelectronics in medicine. Adv. Mater. 34, 2106787 (2022).
Han, M. et al. Three-dimensional piezoelectric polymer microsystems for vibrational energy harvesting, robotic interfaces and biomedical implants. Nat. Electron. 2, 26–35 (2019).
Zhou, Y. et al. Stretchable high-permittivity nanocomposites for epidermal alternating-current electroluminescent displays. ACS Mater. Lett. 1, 511–518 (2019).
Luo, Y. et al. Devising materials manufacturing toward lab-to-fab translation of flexible electronics. Adv. Mater. 32, 2001903 (2020).
Shi, H. H. et al. Sustainable electronic textiles towards scalable commercialization. Nat. Mater. 22, 1294–1303 (2023).
Kiran, D. R. In: Total Quality Management: Key Concepts and Case Studies 391–404 (Elsevier Science, 2017).
Zheng, Y. et al. Environmentally stable and stretchable polymer electronics enabled by surface-tethered nanostructured molecular-level protection. Nat. Nanotechnol. 18, 1175–1184 (2023).
Bierman, A. S. & Tinetti, M. E. Precision medicine to precision care: managing multimorbidity. Lancet 388, 2721–2723 (2016).
Zhou, Z. et al. Sign-to-speech translation using machine-learning-assisted stretchable sensor arrays. Nat. Electron. 3, 571–578 (2020).
Bouton, C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247–250 (2016).
Hou, B. et al. An interactive mouthguard based on mechanoluminescence-powered optical fibre sensors for bite-controlled device operation. Nat. Electron. 5, 682–693 (2022).
Yu, X. et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 (2019).
Bai, H. et al. Stretchable distributed fiber-optic sensors. Science 370, 848–852 (2020).
Kerr, E. A. & Hayward, R. A. Patient-centered performance management: enhancing value for patients and health care systems. JAMA 310, 137–138 (2013).
Rose, N. Personalized medicine: promises, problems and perils of a new paradigm for healthcare. Procedia Soc. Behav. Sci. 77, 341–352 (2013).
Li, Z., Tian, X., Qiu, C.-W. & Ho, J. S. Metasurfaces for bioelectronics and healthcare. Nat. Electron. 4, 382–391 (2021).
Schwalbe, N. & Wahl, B. Artificial intelligence and the future of global health. Lancet 395, 1579–1586 (2020).
Kim, J. et al. Skin-interfaced wireless biosensors for perinatal and paediatric health. Nat. Rev. Bioeng. 1, 631–647 (2023).
Xu, S. et al. Wireless skin sensors for physiological monitoring of infants in low-income and middle-income countries. Lancet Digit. Health 3, e266–e273 (2021).
Acknowledgements
The authors acknowledge the Henry Samueli School of Engineering & Applied Science and the Department of Bioengineering at the University of California, Los Angeles, for their startup support. J.C. acknowledges the Vernroy Makoto Watanabe Excellence in Research Award at the UCLA Samueli School of Engineering, the Office of Naval Research Young Investigator Award (award ID: N00014-24-1-2065), NIH grant (award ID: R01 CA287326), the American Heart Association Innovative Project Award (award ID: 23IPA1054908), the American Heart Association Transformational Project Award (award ID: 23TPA1141360), the American Heart Association’s Second Century Early Faculty Independence Award (award ID: 23SCEFIA1157587), the Brain & Behavior Research Foundation Young Investigator Grant (grant number: 30944), and the NIH National Center for Advancing Translational Science UCLA CTSI (grant number: KL2TR001882).
Author information
Authors and Affiliations
Contributions
J.C. guided the project. J.C. wrote, edited and reviewed the article. J.Y. and S.W. contributed equally to the preparation of this manuscript. T.T. made technical comments on the article. All authors have seen the paper, agree to its content and approve submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Donghee Son and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, J., Wang, S., Tat, T. et al. Motion artefact management for soft bioelectronics. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00175-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00175-4