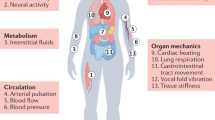
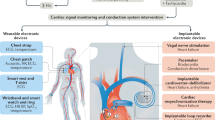
Abstract
The motions of the heart are regulated by electrophysiological signals, which can be monitored and altered by bioelectronic devices for the diagnosis and treatment of cardiovascular diseases. In particular, soft bioelectronic devices, composed of deformable and conductive materials, can be implanted or designed as wearable devices to enable conformal contact with heart tissue or the skin for real-time and precise diagnosis and treatment. In this Review, we discuss the design and materials of soft bioelectronic devices, highlighting their advantages, as compared with rigid bioelectronic devices, in cardiovascular disease management. We examine the engineering and applications of soft implantable bioelectronics, including cardiac mapping devices, cardiac stimulation devices and mechanically assisting devices, as well as wearable soft bioelectronics, such as blood oxygen saturation sensors, heart monitoring devices and transdermal drug delivery systems. Finally, we outline technical challenges and future opportunities for the clinical application of soft bioelectronic devices, such as a wireless power supply, optogenetic control of cardiac motion, bioadhesives for device–tissue interfaces and artificial intelligence-assisted data analysis.
Key points
-
The electrophysiological state of the heart can be monitored and manipulated by bioelectronics for the diagnosis and treatment of cardiovascular disease.
-
Deformable, conductive bioelectronics can be designed to interface with heart tissue and manage cardiovascular disease with high diagnostic accuracy and treatment efficacy.
-
Soft electronic materials, such as conductive polymers, hydrogels, liquid metals and stretchable nanocomposites, enable conformal device–tissue interfaces, addressing the mechanical mismatch of rigid bioelectronic devices.
-
Soft bioelectronics can be engineered as multichannel arrays for 3D cardiac mapping, localized therapy, heart modulation and mechanical control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tirilomis, T. Heart research and ancient Greek medicine. Heart 89, 2 (2003).
DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 106, 434–446 (2010).
Hong, Y. J., Jeong, H., Cho, K. W., Lu, N. & Kim, D. Wearable and implantable devices for cardiovascular healthcare: from monitoring to therapy based on flexible and stretchable electronics. Adv. Funct. Mater. 29, 1808247 (2019).
Tracy, C. M. et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J. Am. Coll. Cardiol. 61, e6–e75 (2013).
Udo, E. O. et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 9, 728–735 (2012).
Lim, Y.-M. et al. Subclinical cardiac perforation by cardiac implantable electronic device leads detected by cardiac computed tomography. BMC Cardiovasc. Disord. 21, 346 (2021).
Addetia, K., Harb, S. C., Hahn, R. T., Kapadia, S. & Lang, R. M. Cardiac implantable electronic device lead-induced tricuspid regurgitation. JACC Cardiovasc. Imaging 12, 622–636 (2019).
Dean, J. & Sulke, N. Pacemaker battery scandal. Br. Med. J. 352, i228 (2016).
Searle, A. & Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 21, 271–283 (2000).
Ma, Z. et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Koo, J. H. et al. Unconventional device and material approaches for monolithic biointegration of implantable sensors and wearable electronics. Adv. Mater. Technol. 5, 2000407 (2020).
Lee, Y. et al. Wearable sensing systems with mechanically soft assemblies of nanoscale materials. Adv. Mater. Technol. 2, 1700053 (2017).
Choi, S., Lee, H., Ghaffari, R., Hyeon, T. & Kim, D.-H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 28, 4203–4218 (2016).
Sunwoo, S.-H., Ha, K.-H., Lee, S., Lu, N. & Kim, D.-H. Wearable and implantable soft bioelectronics: device designs and material strategies. Annu. Rev. Chem. Biomol. Eng. 12, 359–391 (2021).
Wang, C., Wang, C., Huang, Z. & Xu, S. Materials and structures toward soft electronics. Adv. Mater. 30, 1801368 (2018).
Xue, Z., Song, H., Rogers, J. A., Zhang, Y. & Huang, Y. Mechanically‐guided structural designs in stretchable inorganic electronics. Adv. Mater. 32, 1902254 (2020).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Sunwoo, S. H. et al. Chronic and acute stress monitoring by electrophysiological signals from adrenal gland. Proc. Natl Acad. Sci. USA 116, 1146–1151 (2019).
Ha, T. et al. A chest‐laminated ultrathin and stretchable e‐tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. 6, 1900290 (2019).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Kim, D.-H. et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 10, 316–323 (2011).
Gutruf, P. et al. Wireless, battery-free, fully implantable multimodal and multisite pacemakers for applications in small animal models. Nat. Commun. 10, 5742 (2019).
Lim, C. et al. Tissue-like skin–device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 7, eabd3716 (2021).
Liu, S., Rao, Y., Jang, H., Tan, P. & Lu, N. Strategies for body-conformable electronics. Matter 5, 1104–1136 (2022).
Singh, G. & Chanda, A. Mechanical properties of whole-body soft human tissues: a review. Biomed. Mater. 16, 062004 (2021).
Boyd, E. J. & Uttamchandani, D. Measurement of the anisotropy of Young’s modulus in single-crystal silicon. J. Microelectromech. Sys. 21, 243–249 (2012).
Qu, C., Hu, J., Liu, X., Li, Z. & Ding, Y. Morphology and mechanical properties of polyimide films: the effects of UV irradiation on microscale surface. Materials 10, 1329 (2017).
Martín-Sánchez, C. et al. On the stiffness of gold at the nanoscale. ACS Nano 15, 19128–19137 (2021).
Van Houten, E. E. W., Doyley, M. M., Kennedy, F. E., Weaver, J. B. & Paulsen, K. D. Initial in vivo experience with steady-state subzone-based MR elastography of the human breast. J. Magnet. Reson. Imaging 17, 72–85 (2003).
Budday, S. et al. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 46, 318–330 (2015).
Lim, Y.-J., Deo, D., Singh, T. P., Jones, D. B. & De, S. In situ measurement and modeling of biomechanical response of human cadaveric soft tissues for physics-based surgical simulation. Surg. Endosc. 23, 1298–1307 (2009).
Booth, A. J. et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 186, 866–876 (2012).
Holzapfel, G. A., Sommer, G., Gasser, C. T. & Regitnig, P. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am. J. Physiol. Heart Circ. Physiol. 289, H2048–H2058 (2005).
Uffmann, K. et al. In vivo elasticity measurements of extremity skeletal muscle with MR elastography. NMR Biomed. 17, 181–190 (2004).
Zwirner, J., Ondruschka, B., Scholze, M. & Hammer, N. Passive load-deformation properties of human temporal muscle. J. Biomech. 106, 109829 (2020).
Ottenio, M., Tran, D., Ní Annaidh, A., Gilchrist, M. D. & Bruyère, K. Strain rate and anisotropy effects on the tensile failure characteristics of human skin. J. Mech. Behav. Biomed. Mater. 41, 241–250 (2015).
Tang, L., Shang, J. & Jiang, X. Multilayered electronic transfer tattoo that can enable the crease amplification effect. Sci. Adv. 7, abe3778 (2021).
Kim, D. C., Shim, H. J., Lee, W., Koo, J. H. & Kim, D. Material‐based approaches for the fabrication of stretchable electronics. Adv. Mater. 32, 1902743 (2020).
Sunwoo, S.-H. et al. Advances in soft bioelectronics for brain research and clinical neuroengineering. Matter 3, 1923–1947 (2020).
Guan, Y.-S. et al. Air/water interfacial assembled rubbery semiconducting nanofilm for fully rubbery integrated electronics. Sci. Adv. 6, eabb3656 (2020).
Basescu, N. et al. High electrical conductivity in doped polyacetylene. Nature 327, 403–405 (1987).
Worfolk, B. J. et al. Ultrahigh electrical conductivity in solution-sheared polymeric transparent films. Proc. Natl Acad. Sci. USA 112, 14138–14143 (2015).
Modarresi, M. & Zozoulenko, I. Why does solvent treatment increase the conductivity of PEDOT:PSS? Insight from molecular dynamics simulations. Phys. Chem. Chem. Phys. 24, 22073–22082 (2022).
Xia, Y., Zhang, H. & Ouyang, J. Highly conductive PEDOT:PSS films prepared through a treatment with zwitterions and their application in polymer photovoltaic cells. J. Mater. Chem. 20, 9740–9747 (2010).
Ali, M. Z. et al. Single-step treatment to improve conductivity of PEDOT:PSS by hydrobromic acid solution for application of transparent electrode. Org. Electron. 110, 106643 (2022).
Rohtlaid, K. et al. Poly(3,4‐ethylenedioxythiophene):poly(styrene sulfonate)/polyethylene oxide electrodes with improved electrical and electrochemical properties for soft microactuators and microsensors. Adv. Electron. Mater. 5, 1800948 (2019).
Aslani, A. in Polypropylene (ed. Dogan, F.) 219–264 (InTech, 2012).
Nguyen, D. T. & Tumolo, A. Z. Narrowing the field. JACC Clin. Electrophysiol. 5, 78–80 (2019).
Puiggalí-Jou, A., del Valle, L. J. & Alemán, C. Drug delivery systems based on intrinsically conducting polymers. J. Control. Release 309, 244–264 (2019).
Peng, Q. et al. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2, 843–865 (2020).
Mano, N., Yoo, J. E., Tarver, J., Loo, Y.-L. & Heller, A. An electron-conducting cross-linked polyaniline-based redox hydrogel, formed in one step at pH 7.2, wires glucose oxidase. J. Am. Chem. Soc. 129, 7006–7007 (2007).
Shi, Y., Ma, C., Peng, L. & Yu, G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 25, 1219–1225 (2015).
Lu, B. et al. Pure PEDOT:PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Fan, F. R., Tang, W. & Wang, Z. L. Flexible nanogenerators for energy harvesting and self-powered electronics. Adv. Mater. 28, 4283–4305 (2016).
Keplinger, C. et al. Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013).
Morelle, X. P. et al. Highly stretchable and tough hydrogels below water freezing temperature. Adv. Mater. 30, 1801541 (2018).
Si, Y. et al. Ultrahigh-water-content, superelastic, and shape-memory nanofiber-assembled hydrogels exhibiting pressure-responsive conductivity. Adv. Mater. 29, 1700339 (2017).
Liu, Y.-J., Cao, W.-T., Ma, M.-G. & Wan, P. Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl. Mater. Interfaces 9, 25559–25570 (2017).
Liu, T. et al. Triboelectric-nanogenerator-based soft energy-harvesting skin enabled by toughly bonded elastomer/hydrogel hybrids. ACS Nano 12, 2818–2826 (2018).
Lai, J. et al. Recyclable, stretchable and conductive double network hydrogels towards flexible strain sensors. J. Mater. Chem. C. Mater. 6, 13316–13324 (2018).
Liu, X., Wu, D., Wang, H. & Wang, Q. Self-recovering tough gel electrolyte with adjustable supercapacitor performance. Adv. Mater. 26, 4370–4375 (2014).
Xu, Y. et al. Functionalized graphene hydrogel-based high-performance supercapacitors. Adv. Mater. 25, 5779–5784 (2013).
Liu, Q. et al. High-quality graphene ribbons prepared from graphene oxide hydrogels and their application for strain sensors. ACS Nano 9, 12320–12326 (2015).
Han, L. et al. Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv. Funct. Mater. 28, 1704195 (2018).
Nam, J. et al. Supramolecular peptide hydrogel-based soft neural interface augments brain signals through a three-dimensional electrical network. ACS Nano 14, 664–675 (2020).
Fantino, E. et al. 3D printing of conductive complex structures with in situ generation of silver nanoparticles. Adv. Mater. 28, 3712–3717 (2016).
Pardo-Yissar, V., Gabai, R., Shipway, A. N., Bourenko, T. & Willner, I. Gold nanoparticle/hydrogel composites with solvent-switchable electronic properties. Adv. Mater. 13, 1320 (2001).
Lim, C. et al. Stretchable conductive nanocomposite based on alginate hydrogel and silver nanowires for wearable electronics. APL Mater. 7, 031502 (2019).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Lee, C.-J. et al. Ionic conductivity of polyelectrolyte hydrogels. ACS Appl. Mater. Interfaces 10, 5845–5852 (2018).
Wang, S. et al. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose–bentonite coordination interactions. Nat. Commun. 13, 3408 (2022).
Shay, T., Velev, O. D. & Dickey, M. D. Soft electrodes combining hydrogel and liquid metal. Soft Matter 14, 3296–3303 (2018).
Khoshmanesh, K. et al. Liquid metal enabled microfluidics. Lab Chip 17, 974–993 (2017).
Lu, T., Finkenauer, L., Wissman, J. & Majidi, C. Rapid prototyping for soft-matter electronics. Adv. Funct. Mater. 24, 3351–3356 (2014).
Pan, C. et al. Visually imperceptible liquid‐metal circuits for transparent, stretchable electronics with direct laser writing. Adv. Mater. 30, 1706937 (2018).
Deng, B. & Cheng, G. J. Pulsed laser modulated shock transition from liquid metal nanoparticles to mechanically and thermally robust solid–liquid patterns. Adv. Mater. 31, 1807811 (2019).
Park, C. W. et al. Photolithography-based patterning of liquid metal interconnects for monolithically integrated stretchable circuits. ACS Appl. Mater. Interfaces 8, 15459–15465 (2016).
Kim, M., Brown, D. K. & Brand, O. Nanofabrication for all-soft and high-density electronic devices based on liquid metal. Nat. Commun. 11, 1002 (2020).
Li, G., Wu, X. & Lee, D.-W. Selectively plated stretchable liquid metal wires for transparent electronics. Sens. Actu. B Chem. 221, 1114–1119 (2015).
Gannarapu, A. & Gozen, B. A. Freeze-printing of liquid metal alloys for manufacturing of 3D, conductive, and flexible networks. Adv. Mater. Technol. 1, 1600047 (2016).
Tabatabai, A., Fassler, A., Usiak, C. & Majidi, C. Liquid-phase gallium–indium alloy electronics with microcontact printing. Langmuir 29, 6194–6200 (2013).
Boley, J. W., White, E. L., Chiu, G. T. C. & Kramer, R. K. Direct writing of gallium–indium alloy for stretchable electronics. Adv. Funct. Mater. 24, 3501–3507 (2014).
Ladd, C., So, J.-H., Muth, J. & Dickey, M. D. 3D printing of free standing liquid metal microstructures. Adv. Mater. 25, 5081–5085 (2013).
Veerapandian, S. et al. Hydrogen-doped viscoplastic liquid metal microparticles for stretchable printed metal lines. Nat. Mater. 20, 533–540 (2021).
Pan, C. et al. A liquid‐metal–elastomer nanocomposite for stretchable dielectric materials. Adv. Mater. 31, 1900663 (2019).
Cheng, S. et al. Electronic blood vessel. Matter 3, 1664–1684 (2020).
Li, Y. et al. A highly stretchable and permeable liquid metal micromesh conductor by physical deposition for epidermal electronics. ACS Appl. Mater. Interfaces 14, 13713–13721 (2022).
Yan, J. et al. Solution processable liquid metal nanodroplets by surface-initiated atom transfer radical polymerization. Nat. Nanotechnol. 14, 684–690 (2019).
Yang, J. et al. Ultrasoft liquid metal elastomer foams with positive and negative piezopermittivity for tactile sensing. Adv. Funct. Mater. 30, 2002611 (2020).
Yun, G. et al. Liquid metal composites with anisotropic and unconventional piezoconductivity. Matter 3, 824–841 (2020).
Choi, S., Han, S. I., Kim, D., Hyeon, T. & Kim, D.-H. High-performance stretchable conductive nanocomposites: materials, processes, and device applications. Chem. Soc. Rev. 48, 1566–1595 (2019).
Cho, K. W. et al. Soft bioelectronics based on nanomaterials. Chem. Rev. 122, 5068–5143 (2022).
Park, C. et al. Stretchable conductive nanocomposites and their applications in wearable devices. Appl. Phys. Rev. 9, 21312 (2022).
Son, D. et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 13, 1057–1065 (2018).
Sim, K. et al. Fully rubbery integrated electronics from high effective mobility intrinsically stretchable semiconductors. Sci. Adv. 5, eaav5749 (2019).
Wang, K. et al. High‐performance graphene‐fiber‐based neural recording microelectrodes. Adv. Mater. 31, 1805867 (2019).
Vitale, F., Summerson, S. R., Aazhang, B., Kemere, C. & Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 9, 4465–4474 (2015).
Bullara, L. A., McCreery, D. B., Yuen, T. G. H. & Agnew, W. F. A microelectrode for delivery of defined charge densities. J. Neurosci. Meth. 9, 15–21 (1983).
Weiland, J. D., Anderson, D. J. & Humayun, M. S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 49, 1574–1579 (2002).
Cogan, S. F., Guzelian, A. A., Agnew, W. F., Yuen, T. G. H. & McCreery, D. B. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J. Neurosci. Meth. 137, 141–150 (2004).
Venkatraman, S. et al. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Sys. Rehabilitation Eng. 19, 307–316 (2011).
Lu, L. et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. Nano Lett. 19, 1577–1586 (2019).
Lu, Y., Lyu, H., Richardson, A. G., Lucas, T. H. & Kuzum, D. Flexible neural electrode array based-on porous graphene for cortical microstimulation and sensing. Sci. Rep. 6, 33526 (2016).
Park, D.-W. et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5, 5258 (2014).
Chen, R. J. et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl Acad. Sci. USA 100, 4984–4989 (2003).
Chen, K. et al. Printed carbon nanotube electronics and sensor systems. Adv. Mater. 28, 4397–4414 (2016).
Murata, H. et al. High-electrical-conductivity multilayer graphene formed by layer exchange with controlled thickness and interlayer. Sci. Rep. 9, 4068 (2019).
Yetisgin, A. A., Sakar, H., Bermek, H. & Trabzon, L. Production of elastomer-based highly conductive hybrid nanocomposites and treatment with sulfuric acid. J. Polym. Eng. 41, 467–479 (2021).
Mohanta, D., Patnaik, S., Sood, S. & Das, N. Carbon nanotubes: evaluation of toxicity at biointerfaces. J. Pharma. Anal. 9, 293–300 (2019).
Liu, C.-H. & Yu, X. Silver nanowire-based transparent, flexible, and conductive thin film. Nanoscale Res. Lett. 6, 75 (2011).
Della Gaspera, E. et al. Copper-based conductive composites with tailored thermal expansion. ACS Appl. Mater. Interfaces 5, 10966–10974 (2013).
Blasco, E. et al. Fabrication of conductive 3D gold-containing microstructures via direct laser writing. Adv. Mater. 28, 3592–3595 (2016).
You, I. et al. Stretchable e-skin apexcardiogram sensor. Adv. Mater. 28, 6359–6364 (2016).
Wu, H. P. et al. Effect analysis of filler sizes on percolation threshold of isotropical conductive adhesives. Compos. Sci. Technol. 67, 1116–1120 (2007).
Matsuhisa, N. et al. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 16, 834–840 (2017).
Koo, J. H. et al. Recent advances in soft electronic materials for intrinsically stretchable optoelectronic systems. Opto-Electron. Adv. 5, 210131 (2022).
Song, J.-K. et al. Stretchable colour-sensitive quantum dot nanocomposites for shape-tunable multiplexed phototransistor arrays. Nat. Nanotechnol. 17, 849–856 (2022).
Ilami, M., Bagheri, H., Ahmed, R., Skowronek, E. O. & Marvi, H. Materials, actuators, and sensors for soft bioinspired robots. Adv. Mater. 33, 2003139 (2021).
Raza, S. et al. Biosynthesis of silver nanoparticles for the fabrication of non cytotoxic and antibacterial metallic polymer based nanocomposite system. Sci. Rep. 11, 10500 (2021).
Pramanik, P. K., Khastgir, D. & Saha, T. N. Conductive nitrile rubber composite containing carbon fillers: studies on mechanical properties and electrical conductivity. Composites 23, 183–191 (1992).
Khan, T. et al. Insights to low electrical percolation thresholds of carbon-based polypropylene nanocomposites. Carbon 176, 602–631 (2021).
Choi, S. et al. Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 9, 6626–6633 (2015).
Jung, D. et al. Adaptive self‐organization of nanomaterials enables strain‐insensitive resistance of stretchable metallic nanocomposites. Adv. Mater. 34, 2200980 (2022).
Yun, Y. S., Kim, D. H., Kim, B., Park, H. H. & Jin, H.-J. Transparent conducting films based on graphene oxide/silver nanowire hybrids with high flexibility. Synth. Met. 162, 1364–1368 (2012).
Lin, H. et al. Selective fabrication of nanowires with high aspect ratios using a diffusion mixing reaction system for applications in temperature sensing. Anal. Chem. 91, 7346–7352 (2019).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Sunwoo, S. H. et al. Stretchable low-impedance nanocomposite comprised of Ag–Au core–shell nanowires and Pt black for epicardial recording and stimulation. Adv. Mater. Technol. 5, 1900768 (2020).
Wilms, M., Conrad, J., Vasilev, K., Kreiter, M. & Wegner, G. Manipulation and conductivity measurements of gold nanowires. Appl. Surf. Sci. 238, 490–494 (2004).
Maurer, J. H. M., González-García, L., Reiser, B., Kanelidis, I. & Kraus, T. Templated self-assembly of ultrathin gold nanowires by nanoimprinting for transparent flexible electronics. Nano Lett. 16, 2921–2925 (2016).
Wang, J. et al. Near-ideal theoretical strength in gold nanowires containing angstrom scale twins. Nat. Commun. 4, 1742 (2013).
Lim, C. et al. Facile and scalable synthesis of whiskered gold nanosheets for stretchable, conductive, and biocompatible nanocomposites. ACS Nano 16, 10431–10442 (2022).
Cho, S. et al. Fully elastic conductive films from viscoelastic composites. ACS Appl. Mater. Interfaces 9, 44096–44105 (2017).
Moon, G. D. et al. Highly stretchable patterned gold electrodes made of Au nanosheets. Adv. Mater. 25, 2707–2712 (2013).
Lim, G.-H., Lee, N.-E. & Lim, B. Highly sensitive, tunable, and durable gold nanosheet strain sensors for human motion detection. J. Mater. Chem. C Mater 4, 5642–5647 (2016).
Jeong, S., Heo, S., Kang, M. & Kim, H.-J. Mechanical durability enhancement of gold-nanosheet stretchable electrodes for wearable human bio-signal detection. Mater. Des. 196, 109178 (2020).
Lim, G.-H. et al. Fully stretchable and highly durable triboelectric nanogenerators based on gold-nanosheet electrodes for self-powered human-motion detection. Nano Energy 42, 300–306 (2017).
Ryu, J. et al. Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum-air flow batteries. Nat. Commun. 9, 3715 (2018).
Kelly, A. G. et al. Highly conductive networks of silver nanosheets. Small 18, 2105996 (2022).
Shim, H. J., Sunwoo, S., Kim, Y., Koo, J. H. & Kim, D. Functionalized elastomers for intrinsically soft and biointegrated electronics. Adv. Healthc. Mater. 10, 2002105 (2021).
Emig, R. et al. Passive myocardial mechanical properties: meaning, measurement, models. Biophys. Rev. 13, 587–610 (2021).
Dutta, D. et al. Non-invasive assessment of elastic modulus of arterial constructs during cell culture using ultrasound elasticity imaging. Ultrasound Med. Biol. 39, 2103–2115 (2013).
Han, M. et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat. Biomed. Eng. 4, 997–1009 (2020).
Viventi, J. et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl Med. 2, 24ra22 (2010).
van Heerebeek, L. et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113, 1966–1973 (2006).
Lee, W. et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 4, aau2426 (2018).
Wang, S. et al. Intrinsically stretchable electronics with ultrahigh deformability to monitor dynamically moving organs. Sci. Adv. 8, eabl5511 (2023).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Anderson, R. D. et al. On the electrophysiology and mapping of intramural arrhythmic focus. Circ. Arrhythm. Electrophysiol. 15, e010384 (2022).
Cha, G. D. et al. Multifunctional injectable hydrogel for in vivo diagnostic and therapeutic applications. ACS Nano 16, 554–567 (2022).
Xu, L. et al. Materials and fractal designs for 3D multifunctional integumentary membranes with capabilities in cardiac electrotherapy. Adv. Mater. 27, 1731–1737 (2015).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl Med. 8, 344ra86 (2016).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Li, N. et al. Direct powering a real cardiac pacemaker by natural energy of a heartbeat. ACS Nano 13, 2822–2830 (2019).
Ausra, J. et al. Wireless, fully implantable cardiac stimulation and recording with on-device computation for closed-loop pacing and defibrillation. Sci. Adv. 8, eabq7469 (2022).
Oliveira, G. H., Al-Kindi, S. G., G. Bezerra, H. & Costa, M. A. Left ventricular restoration devices. J. Cardiovasc. Transl. Res. 7, 282–291 (2014).
Roche, E. T. et al. Soft robotic sleeve supports heart function. Sci. Transl Med. 9, eaaf3925 (2017).
Han, J., Aranda-Michel, E. & Trumble, D. R. Muscle-powered counterpulsation for untethered, non-blood-contacting cardiac support: a path to destination therapy. IEEE Trans. Biomed. Eng. 67, 3035–3047 (2020).
Davis, I. M. “Round, red globules floating in a crystalline fluid” – Antoni van Leeuwenhoek’s observations of red blood cells and hemocytes. Micron 157, 103249 (2022).
Mendelson, Y. & Ochs, B. D. Noninvasive pulse oximetry utilizing skin reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 35, 798–805 (1988).
Yokota, T. et al. Ultraflexible organic photonic skin. Sci. Adv. 2, e150185 (2016).
Kim, T.-H. et al. Fully stretchable optoelectronic sensors based on colloidal quantum dots for sensing photoplethysmographic signals. ACS Nano 11, 5992–6003 (2017).
Lee, Y. et al. Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system. Sci. Adv. 7, abg9180 (2021).
Kim, J. et al. Miniaturized battery‐free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 27, 1604373 (2017).
De Bacquer, D., De Backer, G., Kornitzer, M. & Blackburn, H. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart 80, 570–577 (1998).
Giancaterino, S., Lupercio, F., Nishimura, M. & Hsu, J. C. Current and future use of insertable cardiac monitors. JACC Clin. Electrophysiol. 4, 1383–1396 (2018).
Cheng, J. et al. Wet‐adhesive elastomer for liquid metal‐based conformal epidermal electronics. Adv. Funct. Mater. 32, 2200444 (2022).
Koo, J. H. et al. Wearable electrocardiogram monitor using carbon nanotube electronics and color-tunable organic light-emitting diodes. ACS Nano 11, 10032–10041 (2017).
Kim, J. et al. A wearable multiplexed silicon nonvolatile memory array using nanocrystal charge confinement. Sci. Adv. 2, e1501101 (2016).
Lee, S. P. et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Med. 1, 2 (2018).
Yin, L. et al. Chest-scale self-compensated epidermal electronics for standard 6-precordial-lead ECG. npj Flex. Electron. 6, 29 (2022).
Chung, H. U. et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 26, 418–429 (2020).
Jung, D. et al. Highly conductive and elastic nanomembrane for skin electronics. Science 373, 1022–1026 (2021).
Rudy, Y. Noninvasive mapping of repolarization with electrocardiographic imaging. J. Am. Heart. Assoc. 10, e021396 (2021).
Rudy, Y. Noninvasive ECG imaging (ECGI): mapping the arrhythmic substrate of the human heart. Int. J. Cardiol. 237, 13–14 (2017).
Pereira, H., Niederer, S. & Rinaldi, C. A. Electrocardiographic imaging for cardiac arrhythmias and resynchronization therapy. Europace 22, 1447–1462 (2020).
Graham, A. J. et al. Evaluation of ECG imaging to map hemodynamically stable and unstable ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 13, e007377 (2020).
Graham, A. J. et al. Simultaneous comparison of electrocardiographic imaging and epicardial contact mapping in structural heart disease. Circ. Arrhythm. Electrophysiol. 12, e007120 (2019).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Aliyar, H. & Schalau, G. Recent developments in silicones for topical and transdermal drug delivery. Ther. Deliv. 6, 827–839 (2015).
Kim, J., Ghaffari, R. & Kim, D.-H. The quest for miniaturized soft bioelectronic devices. Nat. Biomed. Eng. 1, 0049 (2017).
Yoo, S. et al. Wireless power transfer and telemetry for implantable bioelectronics. Adv. Healthc. Mater. 10, 2100614 (2021).
Kim, H. et al. Wide-range robust wireless power transfer using heterogeneously coupled and flippable neutrals in parity-time symmetry. Sci. Adv. 8, eabo4610 (2023).
Ryu, H. et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 12, 4374 (2021).
Hinchet, R. et al. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019).
Waldmann, V., Narayanan, K. & Marijon, E. Electrical injury-triggered ventricular arrhythmia in a patient with a pacemaker: highlighting the importance of cardiac monitoring. Europace 23, 721–721 (2021).
Parsonnet, V., Villanueva, A., Driller, J. & Berstein, A. D. Corrosion of pacemaker electrodes. Pacing Clin. Electrophysiol. 4, 289–295 (1981).
Kong, H. et al. Corrosive behaviour of Amplatzer® devices in experimental and biological environments. Cardiol. Young 12, 260–265 (2002).
Hauser, R. G. et al. High shocking and pacing impedances due to defibrillation lead calcification. J. Interv. Card. Electrophysiol. 58, 253–259 (2020).
Monkhouse, C., Cambridge, A., Chow, A. W. C. & Behar, J. M. High-voltage impedance rise; mechanism and management in patients with transvenous implantable cardioverter-defibrillators: a case series. Eur. Heart J. Case Rep. 3, 1–8 (2019).
Kołodzińska, A. & Kutarski, A. Lead insulation failure, a serious complication: risk factors and management. Kardiol. Pol. 73, 585–591 (2015).
Entcheva, E. & Kay, M. W. Cardiac optogenetics: a decade of enlightenment. Nat. Rev. Cardiol. 18, 349–367 (2021).
Arrenberg, A. B., Stainier, D. Y. R., Baier, H. & Huisken, J. Optogenetic control of cardiac function. Science 330, 971–974 (2010).
Jia, Z. et al. Stimulating cardiac muscle by light. Circ. Arrhythm. Electrophysiol. 4, 753–760 (2011).
Zaglia, T. et al. Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of ChannelRhodopsin-2. Proc. Natl Acad. Sci. USA 112, E4495–E4504 (2015).
Nussinovitch, U. & Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 33, 750–754 (2015).
Vogt, C. C. et al. Systemic gene transfer enables optogenetic pacing of mouse hearts. Cardiovasc. Res. 106, 338–343 (2015).
Bingen, B. O. et al. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc. Res. 104, 194–205 (2014).
Nyns, E. C. A. et al. Optogenetic termination of ventricular arrhythmias in the whole heart: towards biological cardiac rhythm management. Eur. Heart J. 38, ehw574 (2016).
Bruegmann, T., Beiert, T., Vogt, C. C., Schrickel, J. W. & Sasse, P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc. Res. 114, 713–723 (2018).
Nyns, E. C. A. et al. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl Med. 11, aau6447 (2019).
Govorunova, E. G., Cunha, S. R., Sineshchekov, O. A. & Spudich, J. L. Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci. Rep. 6, 33530 (2016).
Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Xu, L. et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 5, 3329 (2014).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Siontis, K. C., Noseworthy, P. A., Attia, Z. I. & Friedman, P. A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 18, 465–478 (2021).
Ribeiro, A. H. et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 11, 1760 (2020).
Attia, Z. I. et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 25, 70–74 (2019).
Attia, Z. I. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394, 861–867 (2019).
Attia, Z. I. et al. Novel bloodless potassium determination using a signal‐processed single‐lead ECG. J. Am. Heart. Assoc. 5, e002746 (2016).
Ko, W.-Y. et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J. Am. Coll. Cardiol. 75, 722–733 (2020).
Tison, G. H. et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 3, 409 (2018).
Sang, M., Kim, K., Shin, J. & Yu, K. J. Ultra-thin flexible encapsulating materials for soft bio-integrated electronics. Adv. Sci. 9, 2202980 (2022).
Sunwoo, S.-H. et al. Stretchable low-impedance conductor with Ag–Au–Pt core–shell–shell nanowires and in situ formed pt nanoparticles for wearable and implantable device. ACS Nano 17, 7550–7561 (2023).
Valentová, H. & Stejskal, J. Mechanical properties of polyaniline. Synth. Met. 160, 832–834 (2010).
Huang, Z., Ji, Z., Feng, Y., Wang, P. & Huang, Y. Flexible and stretchable polyaniline supercapacitor with a high rate capability. Polym. Int. 70, 437–442 (2021).
Zhang, Y. & Rutledge, G. C. Electrical conductivity of electrospun polyaniline and polyaniline-blend fibers and mats. Macromolecules 45, 4238–4246 (2012).
Shoa, T., Mirfakhrai, T. & Madden, J. D. W. Electro-stiffening in polypyrrole films: dependence of Young’s modulus on oxidation state, load and frequency. Synth. Met. 160, 1280–1286 (2010).
Pang, A. L., Arsad, A. & Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: a short review. Polym. Adv. Technol. 32, 1428–1454 (2021).
Huang, Y. et al. Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 11, 518–525 (2015).
Qu, J., Ouyang, L., Kuo, C. & Martin, D. C. Stiffness, strength and adhesion characterization of electrochemically deposited conjugated polymer films. Acta Biomater. 31, 114–121 (2016).
He, H. et al. Enhancement in the mechanical stretchability of PEDOT:PSS films by compounds of multiple hydroxyl groups for their application as transparent stretchable conductors. Macromolecules 54, 1234–1242 (2021).
Lee, J. H. et al. Highly conductive, stretchable, and transparent PEDOT:PSS electrodes fabricated with triblock copolymer additives and acid treatment. ACS Appl. Mater. Interfaces 10, 28027–28035 (2018).
Chen, R. et al. Highly stretchable and fatigue resistant hydrogels with low Young’s modulus as transparent and flexible strain sensors. J. Mater. Chem. C. Mater. 6, 11193–11201 (2018).
Shin, S. R. et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6, 362–372 (2012).
Hsiao, L.-Y. et al. Carbon nanotube-integrated conductive hydrogels as multifunctional robotic skin. Carbon 161, 784–793 (2020).
Chen, S., Wang, H.-Z., Zhao, R.-Q., Rao, W. & Liu, J. Liquid metal composites. Matter 2, 1446–1480 (2020).
Cao, J. et al. Ultra‐robust stretchable electrode for e‐skin: in situ assembly using a nanofiber scaffold and liquid metal to mimic water‐to‐net interaction. InfoMat 4, e12302 (2022).
Xiao, P., Kim, J.-H. & Seo, S. Flexible and stretchable liquid metal electrodes working at sub-zero temperature and their applications. Materials 14, 4313 (2021).
Tas, M. O. et al. Highly stretchable, directionally oriented carbon nanotube/PDMS conductive films with enhanced sensitivity as wearable strain sensors. ACS Appl. Mater. Interfaces 11, 39560–39573 (2019).
Zhang, D. et al. Multi-functional CNT nanopaper polyurethane nanocomposite fabricated by ultrasonic infiltration and dip soaking processes. Compos. B Eng. 182, 107646 (2020).
Herren, B., Saha, M. C., Altan, M. C. & Liu, Y. Development of ultrastretchable and skin attachable nanocomposites for human motion monitoring via embedded 3D printing. Compos. B Eng. 200, 108224 (2020).
Yang, L. et al. Polydopamine-coated graphene as multifunctional nanofillers in polyurethane. RSC Adv. 3, 6377 (2013).
Wang, X. et al. PDMS-based conductive elastomeric composite with 3D reduced graphene oxide conductive network for flexible strain sensor. Compos. Part. A Appl. Sci. Manuf. 161, 107113 (2022).
Zhang, X. M., Yang, X. L. & Wang, K. Y. Conductive graphene/polydimethylsiloxane nanocomposites for flexible strain sensors. J. Mater. Sci. Mater. Electron. 30, 19319–19324 (2019).
Zeranska, K. et al. Graphene-based thermoplastic composites as extremely broadband and frequency-dependent EMI absorbers for multifunctional applications. ACS Appl. Electron. Mater. 4, 4463–4470 (2022).
Li, X. et al. Graphene/thermoplastic polyurethane nanocomposites: surface modification of graphene through oxidation, polyvinyl pyrrolidone coating and reduction. Compos. Part. A Appl. Sci. Manuf. 68, 264–275 (2015).
Zhong, Z., Luo, S., Yang, K., Wu, X. & Ren, T. High-performance anionic waterborne polyurethane/Ag nanocomposites with excellent antibacterial property via in situ synthesis of Ag nanoparticles. RSC Adv. 7, 42296–42304 (2017).
Hyun, D. C. et al. Ordered zigzag stripes of polymer gel/metal nanoparticle composites for highly stretchable conductive electrodes. Adv. Mater. 23, 2946–2950 (2011).
Park, M. et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 7, 803–809 (2012).
Lu, Y. et al. High-performance stretchable conductive composite fibers from surface-modified silver nanowires and thermoplastic polyurethane by wet spinning. ACS Appl. Mater. Interfaces 10, 2093–2104 (2018).
Cheng, Y., Wang, R., Sun, J. & Gao, L. Highly conductive and ultrastretchable electric circuits from covered yarns and silver nanowires. ACS Nano 9, 3887–3895 (2015).
Kim, I. et al. A photonic sintering derived Ag flake/nanoparticle-based highly sensitive stretchable strain sensor for human motion monitoring. Nanoscale 10, 7890–7897 (2018).
Shin, M. et al. Highly stretchable polymer transistors consisting entirely of stretchable device components. Adv. Mater. 26, 3706–3711 (2014).
Mack, S., Meitl, M. A., Baca, A. J., Zhu, Z.-T. & Rogers, J. A. Mechanically flexible thin-film transistors that use ultrathin ribbons of silicon derived from bulk wafers. Appl. Phys. Lett. 88, 213101 (2006).
Oh, H. et al. High density integration of stretchable inorganic thin film transistors with excellent performance and reliability. Nat. Commun. 13, 4963 (2022).
Li et al. An analytical mechanics model for the island-bridge structure of stretchable electronics. Soft Matter 9, 8476–8482 (2013).
Matsubara, K. & Ota, H. Stretchable liquid metal wiring with three-dimentional helical structure. In IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS) 296–298 (IEEE, 2019).
Guan, Y.-S., Zhang, Z., Tang, Y., Yin, J. & Ren, S. (2018). Kirigami-inspired nanoconfined polymer conducting nanosheets with 2000% stretchability. Adv. Mater. 30, 1706390 (2018).
Klodell, C.T. Jr et al. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. J. Thorac. Cardiovasc. Surg. 135, 188–195 (2008).
An, Y.-H. et al. Facilitated transdermal drug delivery using nanocarriers-embedded electroconductive hydrogel coupled with reverse electrodialysis-driven iontophoresis. ACS Nano 14, 4523–4535 (2020).
Ouyang, H. et al. Symbiotic cardiac pacemaker. Nat. Commun. 10, 1821 (2019).
Kim, B. et al. Rapid custom prototyping of soft poroelastic biosensor for simultaneous epicardial recording and imaging. Nat. Commun. 12, 3710 (2021).
Sunwoo, S.-H. et al. Ventricular tachyarrhythmia treatment and prevention by subthreshold stimulation with stretchable epicardial multichannel electrode array. Sci. Adv. 9, eadf6856 (2023).
Cha, G. D., Kang, D., Lee, J. & Kim, D. Bioresorbable electronic implants: history, materials, fabrication, devices, and clinical applications. Adv. Healthc. Mater. 8, 1801660 (2019).
Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R006-D1 and IBS-R006-A1) of the Republic of Korea.
Author information
Authors and Affiliations
Contributions
S.-H.S., S.I.H. and C.S.P. contributed equally to the work. All authors contributed to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Tal Dvir, Igor Efimov and Jia Liu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sunwoo, SH., Han, S.I., Park, C.S. et al. Soft bioelectronics for the management of cardiovascular diseases. Nat Rev Bioeng 2, 8–24 (2024). https://doi.org/10.1038/s44222-023-00102-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00102-z