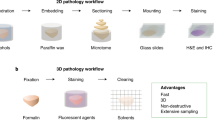
Abstract
Low-cost optical imaging technologies have the potential to reduce inequalities in health care by improving the detection of precancer or early cancer and enabling more effective and less invasive treatment. In this Review, we summarize technologies for in vivo widefield, multi-spectral, endoscopic and high-resolution optical imaging that could offer affordable approaches to improving cancer screening and early detection at the point of care. Additionally, we discuss approaches to slide-free microscopy, including confocal imaging, light-sheet microscopy and phase modulation techniques, that can reduce the infrastructure and expertise needed for definitive cancer diagnosis. We also evaluate how machine learning-based algorithms can improve the accuracy and accessibility of optical imaging systems and provide real-time image analysis. To achieve the potential of optical technologies, developers must ensure that devices are easy to use, the optical technologies must be evaluated in multi-institutional, prospective clinical tests in the intended setting, and the barriers to commercial scale-up in under-resourced markets must be overcome. Therefore, test developers should view the production of simple and effective diagnostic tools that are accessible and affordable for all countries and settings as a central goal of their profession.
Key points
-
Global equity gaps for cancer are growing. Early diagnosis improves patient outcomes, but screening and diagnosis programmes are scarce in low-resource settings, where limitations in infrastructure, human resources, financial resources and/or social resources limit the ability to deliver health care.
-
Advances in consumer-grade imaging tools (such as light-emitting diodes, digital cameras and plastic lenses) have enabled the use of high-performance, low-cost, portable optical imaging systems to visualize cellular, vascular and architectural hallmarks of precancers and early cancers.
-
In vivo optical imaging can improve early detection of cervical, oral, oesophageal, anal and other epithelial cancers, but large studies with commercially available, low-cost devices are needed.
-
To facilitate the adoption of optical imaging techniques in understaffed low-resource settings, technologies to improve cancer screening and early diagnosis must be simple to operate and easy to maintain; therefore, technology developers should emphasize usability throughout the design process.
-
High-quality, slide-free histology can be achieved with low-cost microscopes to improve diagnosis and guide treatments, but large-scale validation of these techniques with standardized staining protocols and commercially available systems is needed.
-
Machine learning can improve imaging performance and reduce the need for human resources by automating image interpretation; however, large, curated image databases from relevant populations are needed for the development and validation of portable algorithms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wild, C. P., Weiderpass, E. & Stewart, B. W. (eds) World Cancer Report: Cancer Research for Cancer Prevention (International Agency for Research on Cancer, 2020).
American Association for Cancer Research. Cancer Disparities Progress Report (AACR, 2022).
Pramesh, C. S. et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat. Med. 28, 649–657 (2022).
Mitchell, E. et al. Cancer healthcare disparities among African Americans in the United States. J. Natl Med. Assoc. 114, 236–250 (2022).
van Zyl, C., Badenhorst, M., Hanekom, S. & Heine, M. Unravelling ‘low-resource settings’: a systematic scoping review with qualitative content analysis. BMJ Glob. Health 6, e005190 (2021).
World Health Organization. Saving Lives, Spending Less: A Strategic Response to Noncommunicable Diseases (WHO, 2018).
World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2030 https://iris.who.int/bitstream/handle/10665/94384/9789241506236_eng.pdf;jsessionid=499437100E28C25D028AD5B112AFBF92?sequence=1 (2013).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Ryan, B. M. & Faupel-Badger, J. M. The hallmarks of premalignant conditions: a molecular basis for cancer prevention. Semin. Oncol. 43, 22–35 (2016).
Arens, C., Betz, C., Kraft, M. & Voigt-Zimmermann, S. Narrow band imaging for early diagnosis of epithelial dysplasia and microinvasive tumors in the upper aerodigestive tract. HNO 65, 5–12 (2017).
Rogalla, S. & Contag, C. H. Early cancer detection at the epithelial surface. Cancer J. 21, 179–187 (2015).
Kundrod, K. A. et al. Advances in technologies for cervical cancer detection in low-resource settings. Expert Rev. Mol. Diagn. 19, 695–714 (2019).
Tian, F., Hu, J. & Yang, W. GEOMScope: large field‐of‐view 3D lensless microscopy with low computational complexity. Laser Photon Rev. 15, 2100072 (2021).
Lim, S. et al. Transnasal endoscopy: moving from endoscopy to the clinical outpatient–blue sky thinking in oesophageal testing. Frontline Gastroenterol. 13, e65–e71 (2022).
Birur, N. P. et al. Field validation of deep learning based point-of-care device for early detection of oral malignant and potentially malignant disorders. Sci. Rep. 12, 14283 (2022).
Bhowmik, A. et al. Portable, handheld, and affordable blood perfusion imager for screening of subsurface cancer in resource-limited settings. Proc. Natl Acad. Sci. USA 119, e2026201119 (2022).
Liu, Y., Rollins, A. M., Levenson, R. M., Fereidouni, F. & Jenkins, M. W. Pocket MUSE: an affordable, versatile and high-performance fluorescence microscope using a smartphone. Commun. Biol. 4, 334 (2021).
Glaser, A. K. et al. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat. Biomed. Eng. 1, 0084 (2017).
Perrin, L., Bayarmagnai, B. & Gligorijevic, B. Frontiers in intravital multiphoton microscopy of cancer. Cancer Rep. 3, e1192 (2020).
Kim, D. H., Kim, S. W. & Hwang, S. H. Autofluorescence imaging to identify oral malignant or premalignant lesions: systematic review and meta‐analysis. Head Neck 42, 3735–3743 (2020).
Jin, L. et al. Deep learning extended depth-of-field microscope for fast and slide-free histology. Proc. Natl Acad. Sci. USA 117, 33051–33060 (2020).
Brenes, D. et al. Multi-task network for automated analysis of high-resolution endomicroscopy images to detect cervical precancer and cancer. Comput. Med. Imaging Graph. 97, 102052 (2022).
Mueller, J. et al. Portable Pocket colposcopy performs comparably to standard-of-care clinical colposcopy using acetic acid and Lugol’s iodine as contrast mediators: an investigational study in Peru. BJOG 125, 1321–1329 (2018).
Kelly, H. et al. Diagnostic accuracy of cervical cancer screening strategies for high-grade cervical intraepithelial neoplasia (CIN2+/CIN3+) among women living with HIV: a systematic review and meta-analysis. EClinicalMedicine 53, 101645 (2022).
Habinshuti, P. et al. Factors associated with loss to follow-up among cervical cancer patients in Rwanda. Ann. Glob. Health 86, 117 (2020).
Mumba, J. M. et al. Cervical cancer diagnosis and treatment delays in the developing world: evidence from a hospital-based study in Zambia. Gynecol. Oncol. Rep. 37, 100784 (2021).
Warnakulasuriya, S. & Kerr, A. R. Oral cancer screening: past, present, and future. J. Dent. Res. 100, 1313–1320 (2021).
Reich, O. & Pickel, H. 200 years of diagnosis and treatment of cervical precancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 165–171 (2020).
Wagner, A. et al. Systematic review on optical diagnosis of early gastrointestinal neoplasia. J. Clin. Med. 10, 2794 (2021).
Akarsu, M. & Akarsu, C. Evaluation of new technologies in gastrointestinal endoscopy. JSLS 22, e2017 (2018).
Bhat, Y. M. et al. High-definition and high-magnification endoscopes. Gastrointest. Endosc. 80, 919–927 (2014).
Prendiville, W. & Sankaranarayanan, R. Colposcopy and Treatment of Cervical Precancer (International Agency for Research on Cancer, 2017).
Cherry, K. D. et al. Autofluorescence imaging to monitor the progression of oral potentially malignant disorders. Cancer Prev. Res. 12, 791–800 (2019).
Yang, E. C. et al. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 670–681 (2018).
Mazur, M. et al. In vivo imaging-based techniques for early diagnosis of oral potentially malignant disorders — systematic review and meta-analysis. Int. J. Env. Res. Public Health 18, 11775 (2021).
Mendonca, P. et al. Non-invasive imaging of oral potentially malignant and malignant lesions: a systematic review and meta-analysis. Oral. Oncol. 130, 105877 (2022).
Parra, S. G. et al. Low-cost, high-resolution imaging for detecting cervical precancer in medically-underserved areas of Texas. Gynecol. Oncol. 154, 558–564 (2019).
Lin, L. & Wang, L. V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022).
Yang, L. et al. Research progress on the application of optical coherence tomography in the field of oncology. Front. Oncol. 12, 953934 (2022).
Ilie, M. et al. Current and future applications of confocal laser scanning microscopy imaging in skin oncology (Review). Oncol. Lett. 17, 4102–4111 (2019).
Glover, B., Teare, J. & Patel, N. The status of advanced imaging techniques for optical biopsy of colonic polyps. Clin. Transl. Gastroenterol. 11, e00130 (2020).
Villard, A. et al. Confocal laser endomicroscopy and confocal microscopy for head and neck cancer imaging: recent updates and future perspectives. Oral. Oncol. 127, 105826 (2022).
Ramani, R. S. et al. Confocal microscopy in oral cancer and oral potentially malignant disorders: a systematic review. Oral. Dis. https://doi.org/10.1111/odi.14291 (2022).
Ring, H. C., Israelsen, N. M., Bang, O., Haedersdal, M. & Mogensen, M. Potential of contrast agents to enhance in vivo confocal microscopy and optical coherence tomography in dermatology: a review. J. Biophotonics 12, e201800462 (2019).
Belykh, E. et al. Molecular imaging of glucose metabolism for intraoperative fluorescence guidance during glioma surgery. Mol. Imaging Biol. 23, 586–596 (2021).
Obeidy, P., Tong, P. L. & Weninger, W. Research techniques made simple: two-photon intravital imaging of the skin. J. Invest. Dermatol. 138, 720–725 (2018).
Steinberg, I. et al. Photoacoustic clinical imaging. Photoacoustics 14, 77–98 (2019).
Wilson, M. L. et al. Access to pathology and laboratory medicine services: a crucial gap. Lancet 391, 1927–1938 (2018).
Hu, L. et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J. Natl Cancer Inst. 111, 923–932 (2019).
World Health Organization. WHO Cervical Cancer Elimination Initiative: From Call to Action to Global Movement (WHO, 2023).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Herrick, T. et al. Acting on the call for cervical cancer elimination: planning tools for low- and middle- income countries to increase the coverage and effectiveness of screening and treatment. BMC Health Serv. Res. 22, 1246 (2022).
Effah, K. et al. A revolution in cervical cancer prevention in Ghana. Ecancermedicalscience 16, ed123 (2022).
Ribeiro, A. et al. Rethinking cervical cancer screening in Brazil post COVID-19: a global opportunity to adopt higher impact strategies. Cancer Prev. Res. 14, 919–926 (2021).
Olubodun, T. et al. Barriers and recommendations for a cervical cancer screening program among women in low-resource settings in Lagos Nigeria: a qualitative study. BMC Public Health 22, 1906 (2022).
Perkins, R. B. et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low. Genit. Tract. Dis. 24, 102–131 (2020).
Vidhubala, E. et al. Loss to follow-up after initial screening for cervical cancer: a qualitative exploration of barriers in Southern India. Cancer Res. Stats. Treat. 3, 700 (2020).
Khozaim, K. et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int. J. Gynecol. Obstet. 124, 12–18 (2014).
World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention 2nd Edition https://www.who.int/publications/i/item/9789240030824 (2021).
Bogdanova, A., Andrawos, C. & Constantinou, C. Cervical cancer, geographical inequalities, prevention and barriers in resource depleted countries (Review). Oncol. Lett. 23, 113 (2022).
Søfteland, S. et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int. J. Gynecol. Obstet. 153, 190–199 (2021).
Peterson, C., Rose, D., Mink, J. & Levitz, D. Real-time monitoring and evaluation of a visual-based cervical cancer screening program using a decision support job aid. Diagnostics 6, 20 (2016).
Goldstein, A. et al. Assessing the feasibility of a rapid, high-volume cervical cancer screening programme using HPV self-sampling and digital colposcopy in rural regions of Yunnan, China. BMJ Open 10, e035153 (2020).
Gallay, C. et al. Cervical cancer screening in low-resource settings: a smartphone image application as an alternative to colposcopy. Int. J. Womens Health 9, 455–461 (2017).
Kudva, V., Prasad, K. & Guruvare, S. Andriod device-based cervical cancer screening for resource-poor settings. J. Digit. Imaging 31, 646–654 (2018).
Mueller, J. L. et al. International image concordance study to compare a point-of-care tampon colposcope with a standard-of-care colposcope. J. Low. Genit. Tract. Dis. 21, 112–119 (2017).
Dayal, U. et al. Comparison of the AV Magnivisualizer device with colposcopy to detect cervical intraepithelial neoplasia using the Swede scoring system. Int. J. Gynecol. Obstet. 147, 219–224 (2019).
Kallner, H. K. et al. Diagnostic colposcopic accuracy by the gynocular and a stationary colposcope. Int. J. Technol. Assess. Health Care 31, 181–187 (2015).
Nessa, A. et al. Evaluation of the accuracy in detecting cervical lesions by nurses versus doctors using a stationary colposcope and Gynocular in a low-resource setting. BMJ Open. 4, e005313 (2014).
Tanaka, Y. et al. Histologic correlation between smartphone and coloposcopic findings in patients with abnormal cervical cytology: experiences in a tertiary referral hospital. Am. J. Obstet. Gynecol. 221, 241.e1–241.e6 (2019).
Tran, P. L. et al. Performance of smartphone-based digital images for cervical cancer screening in a low-resource context. Int. J. Technol. Assess. Health Care 34, 337–342 (2018).
Asgary, R. et al. Evaluating smartphone strategies for reliability, reproducibility, and quality of VIA for cervical cancer screening in the Shiselweni region of Eswatini: a cohort study. PLoS Med. 17, e1003378 (2020).
Mink, J. & Peterson, C. MobileODT: a case study of a novel approach to an mHealth-based model of sustainable impact. Mhealth 2, 12 (2016).
Lam, C. T. et al. Design of a novel low cost point of care tampon (POCkeT) colposcope for use in resource limited settings. PLoS One 10, e0135869 (2015).
Hariprasad, R. & Mehrotra, R. Pocket colposcope: could it improve attendance and increase access to cervical cancer screening programmes? Expert Rev. Anticancer. Ther. 18, 603–605 (2018).
Habtemariam, L. W., Zewde, E. T. & Simegn, G. L. Cervix type and cervical cancer classification system using deep learning techniques. Med. Devices 15, 163–176 (2022).
Guo, P. et al. Ensemble deep learning for cervix image selection toward improving reliability in automated cervical precancer screening. Diagnostics 10, 451 (2020).
Desai, K. T. et al. The development of “automated visual evaluation” for cervical cancer screening: the promise and challenges in adapting deep‐learning for clinical testing. Int. J. Cancer 150, 741–752 (2022).
Pal, A. et al. Deep metric learning for cervical image classification. IEEE Access. 9, 53266–53275 (2021).
Xue, Z. et al. A demonstration of automated visual evaluation of cervical images taken with a smartphone camera. Int. J. Cancer 147, 2416–2423 (2020).
Ahmed, S. R. et al. Reproducible and clinically translatable deep neural networks for cervical screening. Preprint at medRxiv https://doi.org/10.1101/2022.12.17.22282984 (2022).
Xue, Z. et al. A deep clustering method for analyzing uterine cervix images across imaging devices. In Proc. 2021 IEEE 34th International Symposium on Computer-Based Medical Systems (CBMS) 527–532 (IEEE, 2021).
Grant, B. D. et al. A mobile-phone based high-resolution microendoscope to image cervical precancer. PLoS One 14, e0211045 (2019).
Parra, S. et al. Development of a single-board computer high-resolution microendoscope (PiHRME) to detect cervical cancer in low-resource settings. J. Glob. Oncol. 2, 7s–7s (2016).
Quang, T. et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia. Gastrointest. Endosc. 84, 834–841 (2016).
Hunt, B. et al. Diagnosing cervical neoplasia in rural Brazil using a mobile van equipped with in vivo microscopy: a cluster-randomized community trial. Cancer Prev. Res. 11, 359–370 (2018).
Pantano, N. et al. Is proflavine exposure associated with disease progression in women with cervical dysplasia? A brief report. Photochem. Photobiol. 94, 1308–1313 (2018).
Hunt, B. et al. Cervical lesion assessment using real‐time microendoscopy image analysis in Brazil: the CLARA study. Int. J. Cancer 149, 431–441 (2021).
Sheikhzadeh, F. et al. Quantification of confocal fluorescence microscopy for the detection of cervical intraepithelial neoplasia. Biomed. Eng. Online 14, 96 (2015).
Tang, Y. et al. In vivo imaging of cervical precancer using a low-cost and easy-to-use confocal microendoscope. Biomed. Opt. Express. 11, 269–280 (2020).
Zeng, X. et al. Ultrahigh-resolution optical coherence microscopy accurately classifies precancerous and cancerous human cervix free of labeling. Theranostics 8, 3099–3110 (2018).
Ma, Y. et al. Computer-aided diagnosis of label-free 3-D optical coherence microscopy images of human cervical tissue. IEEE Trans. Biomed. Eng. 66, 2447–2456 (2019).
Pouli, D. et al. Label-free, high-resolution optical metabolic imaging of human cervical precancers reveals potential for intraepithelial neoplasia diagnosis. Cell Rep. Med. 1, 100017 (2020).
Gallwas, J. et al. Detection of cervical intraepithelial neoplasia by using optical coherence tomography in combination with microscopy. J. Biomed. Opt. 22, 016013 (2017).
Coole, J. B. et al. Development of a multimodal mobile colposcope for real-time cervical cancer detection. Biomed. Opt. Express 13, 5116 (2022).
Motlokwa, P. K. et al. Disparities in oral cancer stage at presentation in a high HIV prevalence setting in sub-Saharan Africa. JCO Glob. Oncol. 8, e2100439 (2022).
Stanford-Moore, G. et al. Interaction between known risk factors for head and neck cancer and socioeconomic status: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 29, 863–873 (2018).
Gupta, A., Sonis, S., Uppaluri, R., Bergmark, R. W. & Villa, A. Disparities in oral cancer screening among dental professionals: NHANES 2011–2016. Am. J. Prev. Med. 57, 447–457 (2019).
Shabani, S., Turner, K., Nichols, A. C., Wang, X. & Patel, K. B. A review of health care disparities in head and neck squamous cell carcinomas. J. Health Care Poor Underserved 33, 478–491 (2022).
Birur, N. P. et al. Role of community health worker in a mobile health program for early detection of oral cancer. Indian J. Cancer 56, 107 (2019).
Basu, P. et al. A pilot study to evaluate home-based screening for the common non-communicable diseases by a dedicated cadre of community health workers in a rural setting in India. BMC Public Health 19, 14 (2019).
Sankaranarayanan, R. et al. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer 88, 664–673 (2000).
Sankaranarayanan, R. et al. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral. Oncol. 49, 314–321 (2013).
Cheung, L. C. et al. Risk-based selection of individuals for oral cancer screening. J. Clin. Oncol. 39, 663–674 (2021).
Uthoff, R. D. et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS One 13, e0207493 (2018).
Maher, N. G. et al. In vivo confocal microscopy for the oral cavity: current state of the field and future potential. Oral. Oncol. 54, 28–35 (2016).
James, B. L. et al. Validation of a point-of-care optical coherence tomography device with machine learning algorithm for detection of oral potentially malignant and malignant lesions. Cancers 13, 3583 (2021).
Simonato, L. E., Tomo, S., Scarparo Navarro, R. & Balbin Villaverde, A. G. J. Fluorescence visualization improves the detection of oral, potentially malignant, disorders in population screening. Photodiagnosis Photodyn. Ther. 27, 74–78 (2019).
Chiang, T.-E. et al. Comparative evaluation of autofluorescence imaging and histopathological investigation for oral potentially malignant disorders in Taiwan. Clin. Oral. Investig. 23, 2395–2402 (2019).
Lima, I. F. P., Brand, L. M., de Figueiredo, J. A. P., Steier, L. & Lamers, M. L. Use of autofluorescence and fluorescent probes as a potential diagnostic tool for oral cancer: a systematic review. Photodiagnosis Photodyn. Ther. 33, 102073 (2021).
Cicciù, M. et al. Early diagnosis on oral and potentially oral malignant lesions: a systematic review on the VELscope® fluorescence method. Dent. J. 7, 93 (2019).
Moffa, A. et al. Accuracy of autofluorescence and chemiluminescence in the diagnosis of oral dysplasia and carcinoma: a systematic review and meta-analysis. Oral. Oncol. 121, 105482 (2021).
Tiwari, L., Kujan, O. & Farah, C. S. Optical fluorescence imaging in oral cancer and potentially malignant disorders: a systematic review. Oral. Dis. 26, 491–510 (2020).
Pierce, M. C. et al. Accuracy of in vivo multimodal optical imaging for detection of oral neoplasia. Cancer Prev. Res. 5, 801–809 (2012).
Colón-López, V. et al. Anal cancer risk among people with HIV infection in the United States. J. Clin. Oncol. 36, 68 (2018).
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141, 664–670 (2017).
Palefsky, J. M. et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N. Engl. J. Med. 386, 2273–2282 (2022).
Albuquerque, A., Rios, E. & Schmitt, F. Recommendations favoring anal cytology as a method for anal cancer screening: a systematic review. Cancers 11, 1942 (2019).
Clarke, M. A. & Wentzensen, N. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol. 126, 447–460 (2018).
Richel, O., Prins, J. M. & de Vries, H. J. C. Screening for anal cancer precursors: what is the learning curve for high-resolution anoscopy? AIDS 28, 1376–1377 (2014).
Silvera, R. et al. The other side of screening: predictors of treatment and follow-up for anal precancers in a large health system. AIDS 35, 2157–2162 (2021).
Han, C., Huangfu, J., Lai, L. L. & Yang, C. A wide field-of-view scanning endoscope for whole anal canal imaging. Biomed. Opt. Express 6, 607 (2015).
Lai, L. L. et al. Feasibility and safety study of a high resolution wide field-of-view scanning endoscope for circumferential intraluminal intestinal imaging. Sci. Rep. 11, 3544 (2021).
Brenes, D. et al. Automated in vivo high-resolution imaging to detect hpv-associated anal precancer in persons living with HIV. Clin. Transl. Gastroenterol. 14, e00558 (2022).
Ferlay, J. et al. Cancer statistics for the year 2020: an overview. Int. J. Cancer 149, 778–789 (2021).
Săftoiu, A. et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 52, 293–304 (2020).
Zhu, H. et al. Esophageal cancer in China: practice and research in the new era. Int. J. Cancer 152, 1741–1751 (2022).
Waljee, A. K. et al. Artificial intelligence and machine learning for early detection and diagnosis of colorectal cancer in sub-Saharan Africa. Gut 71, 1259–1265 (2022).
Moon, Y. et al. Cost-effective smartphone-based articulable endoscope systems for developing countries: instrument validation study. JMIR Mhealth Uhealth 8, e17057 (2020).
Mwachiro, M. et al. Gastrointestinal endoscopy capacity in Eastern Africa. Endosc. Int. Open 09, E1827–E1836 (2021).
Grant, R. K., Brindle, W. M., Robertson, A. R., Kalla, R. & Plevris, J. N. Unsedated transnasal endoscopy: a safe, well-tolerated and accurate alternative to standard diagnostic peroral endoscopy. Dig. Dis. Sci. 67, 1937–1947 (2022).
Sharma, G. et al. Smartphone‐based multimodal tethered capsule endoscopic platform for white‐light, narrow‐band, and fluorescence/autofluorescence imaging. J. Biophotonics 14, e202000324 (2021).
Kim, Y. et al. A portable smartphone-based laryngoscope system for high-speed vocal cord imaging of patients with throat disorders: instrument validation study. JMIR Mhealth Uhealth 9, e25816 (2021).
Ozcan, A. & McLeod, E. Lensless imaging and sensing. Annu. Rev. Biomed. Eng. 18, 77–102 (2016).
Shin, J. et al. A minimally invasive lens-free computational microendoscope. Sci. Adv. 5, eaaw5595 (2019).
Fleming, K. A. et al. An essential pathology package for low- and middle-income countries. Am. J. Clin. Pathol. 147, 15–32 (2016).
Reiche, M. A. et al. Imaging Africa: a strategic approach to optical microscopy training in Africa. Nat. Methods 18, 847–855 (2021).
Junaid, M. et al. Toluidine blue: yet another low cost method for screening oral cavity tumour margins in third world countries. J. Pak. Med. Assoc. 63, 835–837 (2013).
Costa, C. et al. Use of a low-cost telecytopathology method for remote assessment of thyroid FNAs. Cancer Cytopathol. 126, 767–772 (2018).
Jiang, P. et al. Development of automatic portable pathology scanner and its evaluation for clinical practice. J. Digit. Imaging 36, 1110–1122 (2023).
Coulibaly, J. T. et al. High sensitivity of mobile phone microscopy screening for schistosoma haematobium in Azaguié, Côte d’Ivoire. Am. J. Trop. Med. Hyg. 108, 41–43 (2023).
Xu, K. et al. A novel digital algorithm for identifying liver steatosis using smartphone-captured images. Transpl. Direct 8, e1361 (2022).
Cheng, S. et al. Robust whole slide image analysis for cervical cancer screening using deep learning. Nat. Commun. 12, 5639 (2021).
Sornapudi, S. et al. DeepCIN: attention-based cervical histology image classification with sequential feature modeling for pathologist-level accuracy. J. Pathol. Inf. 11, 40 (2020).
Liu, Y., Levenson, R. M. & Jenkins, M. W. Slide over: advances in slide-free optical microscopy as drivers of diagnostic pathology. Am. J. Pathol. 192, 180–194 (2022).
Fereidouni, F. et al. Microscopy with ultraviolet surface excitation for rapid slide-free histology. Nat. Biomed. Eng. 1, 957–966 (2017).
Qorbani, A. et al. Microscopy with ultraviolet surface excitation (MUSE): a novel approach to real-time inexpensive slide-free dermatopathology. J. Cutan. Pathol. 45, 498–503 (2018).
Zhu, W. et al. Smartphone epifluorescence microscopy for cellular imaging of fresh tissue in low-resource settings. Biomed. Opt. Express 11, 89 (2020).
Reder, N. P. et al. Open-top light-sheet microscopy image atlas of prostate core needle biopsies. Arch. Pathol. Lab. Med. 143, 1069–1075 (2019).
Chen, Y. et al. Rapid pathology of lumpectomy margins with open-top light-sheet (OTLS) microscopy. Biomed. Opt. Express 10, 1257 (2019).
Xie, W. et al. Diagnosing 12 prostate needle cores within an hour of biopsy via open-top light-sheet microscopy. J. Biomed. Opt. 25, 126502 (2020).
Barner, L. A., Glaser, A. K., Huang, H., True, L. D. & Liu, J. T. C. Multi-resolution open-top light-sheet microscopy to enable efficient 3D pathology workflows. Biomed. Opt. Express 11, 6605 (2020).
Pitrone, P. G. et al. OpenSPIM: an open-access light-sheet microscopy platform. Nat. Methods 10, 598–599 (2013).
Hedde, P. N. miniSPIM — a miniaturized light-sheet microscope. ACS Sens. 6, 2654–2663 (2021).
Schiffhauer, L. M. et al. Confocal microscopy of unfixed breast needle core biopsies: a comparison to fixed and stained sections. BMC Cancer 9, 265 (2009).
Torres, R. et al. Initial evaluation of rapid, direct-to-digital prostate biopsy pathology. Arch. Pathol. Lab. Med. 145, 583–591 (2021).
Liang, C. et al. A highly potent ruthenium(II)-sonosensitizer and sonocatalyst for in vivo sonotherapy. Nat. Commun. 12, 5001 (2021).
Mohammadi, S. Phototherapy and sonotherapy of melanoma cancer cells using nanoparticles of selenium-polyethylene glycol-curcumin as a dual-mode sensitizer. J. Biomed. Phys. Eng. 10, 597–606 (2020).
Buzzá, H. H. et al. Overall results for a national program of photodynamic therapy for basal cell carcinoma: a multicenter clinical study to bring new techniques to social health care. Cancer Control https://doi.org/10.1177/1073274819856885 (2019).
Inada, N. M. et al. Long term effectiveness of photodynamic therapy for CIN treatment. Pharmaceuticals 12, 107 (2019).
de Castro, C. A., Lombardi, W., Stringasci, M. D., Bagnato, V. S. & Inada, N. M. High-risk HPV clearance and CIN 3 treated with MAL-PDT: a case report. Photodiagnosis Photodyn. Ther. 31, 101937 (2020).
Saini, R., Lee, N., Liu, K. & Poh, C. Prospects in the application of photodynamic therapy in oral cancer and premalignant lesions. Cancers 8, 83 (2016).
Unanyan, A. et al. Efficacy of photodynamic therapy in women with HSIL, LSIL and early stage squamous cervical cancer: a systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 36, 102530 (2021).
Palamountain, K. M. et al. Perspectives on introduction and implementation of new point-of-care diagnostic tests. J. Infect. Dis. 205, S181–S190 (2012).
Mugambi, M. L., Peter, T., F Martins, S. & Giachetti, C. How to implement new diagnostic products in low-resource settings: an end-to-end framework. BMJ Glob. Health 3, e000914 (2018).
Euliano, E. M., Sklavounos, A. A., Wheeler, A. R. & McHugh, K. J. Translating diagnostics and drug delivery technologies to low-resource settings. Sci. Transl. Med. 14, eabm1732 (2022).
Cocco, P., Ayaz-Shah, A., Messenger, M. P., West, R. M. & Shinkins, B. Target product profiles for medical tests: a systematic review of current methods. BMC Med. 18, 119 (2020).
Sharma, P. et al. The American Society for Gastrointestinal Endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on imaging in Barrett’s esophagus. Gastrointest. Endosc. 76, 252–254 (2012).
Mugambi, M., Palamountain, K., Gallarda, J. & Drain, P. Exploring the case for a global alliance for medical diagnostics initiative. Diagnostics 7, 8 (2017).
Niemeier, D., Gombachika, H. & Richards-Kortum, R. How to transform the practice of engineering to meet global health needs. Science 345, 1287–1290 (2014).
Olympus Corporation. Olympus CF Type Q160ZL/I advanced power zoom. Olympus–Uralendomed http://www.olympus-ural.ru/files/CFQ160ZL_I.pdf (2016).
Li, X., He, S. & Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 19, 12 (2020).
Kohli, D. R. & Baillie, J. in Clinical Gastrointestinal Endoscopy 3rd edn (eds Chandrasekhara, V. et al.) Ch. 3, 24–31.e2 (Elsevier, 2019).
World Health Organization. Guide to Cancer Early Diagnosis https://apps.who.int/iris/handle/10665/254500 (2017).
World Health Organization. Tackling NCDs: ‘Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases https://apps.who.int/iris/handle/10665/259232 (2017).
World Health Organization. The Selection and Use of Essential In Vitro Diagnostics: Report of the Third Meeting of the Who Strategic Advisory Group of Experts on In Vitro Diagnostics, 2020 (Including the Third Who Model List of Essential In Vitro Diagnostics) (WHO Technical Report Series, 2021).
Huckle, D. Point-of-care diagnostics: an advancing sector with nontechnical issues. Expert Rev. Mol. Diagnostics 8, 679–688 (2008).
Sinha, S. R. & Barry, M. Health technologies and innovation in the global health arena. N. Engl. J. Med. 365, 779–782 (2011).
de Oliveira, C. M. et al. HPV testing for cervical cancer screening in Mozambique: challenges and recommendations.J. Glob. Health Rep. 6, e2022007 (2022).
Land, K. J., Boeras, D. I., Chen, X.-S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019).
Ongaro, A. E. et al. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab. Chip 22, 3122–3137 (2022).
Landes, S. J., McBain, S. A. & Curran, G. M. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 280, 112513 (2019).
Bauer, M. S., Damschroder, L., Hagedorn, H., Smith, J. & Kilbourne, A. M. An introduction to implementation science for the non-specialist. BMC Psychol. 3, 32 (2015).
Verbakel, J. Y. et al. Common evidence gaps in point-of-care diagnostic test evaluation: a review of horizon scan reports. BMJ Open 7, e015760 (2017).
Korte, B. J., Rompalo, A., Manabe, Y. C. & Gaydos, C. A. Overcoming challenges with the adoption of point-of-care testing. Point Care 19, 77–83 (2020).
Acknowledgements
The authors gratefully acknowledge the contributions of M. Bond and C. Hicheri with the preparation of figures. This research was supported, in part, by the National Cancer Institute of the National Institutes of Health (NIH) under award number R01CA251911 and through the National Academy of Sciences, United States Agency for International Development (Partnerships for Enhanced Engagement in Research, Cooperative Agreement AID-OAA-A-11-00012).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Patrick Kafui Akakpo, Fernando Schmitt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cervical Precancer Planning Tool: https://www.path.org/resources/cervical-precancer-planning-tool-excel-model/
Global Health Expenditure Database: https://apps.who.int/nha/database/country_profile/Index/en
Invention Education Toolkit: https://ive-toolkit.org
Medical Imaging and Data Resource Center: https://data.midrc.org/
OpenSPIM: http://openspim.org/
Screening tests recommended in the USA: https://www.cancer.gov/about-cancer/screening/screening-tests
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Richards-Kortum, R., Lorenzoni, C., Bagnato, V.S. et al. Optical imaging for screening and early cancer diagnosis in low-resource settings. Nat Rev Bioeng 2, 25–43 (2024). https://doi.org/10.1038/s44222-023-00135-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00135-4