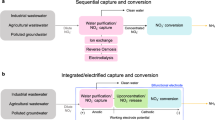
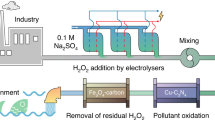
Abstract
Extracting uranium from uranium mine wastewater is highly important from both the environmental protection and the resource preservation perspectives. However, conventional adsorption methods and zero valent iron-induced reductive precipitation methods have intrinsic limitations. Here we propose a spontaneous electrochemical method that spatially decouples the uranium–adsorption–reduction reactions and the iron oxidation reaction, enabling stable and efficient uranium extraction with net electrical energy output. U(VI) species are firstly adsorbed on a carbonaceous electrode, and subsequently reduced by electrons derived from iron oxidation. Using simulated wastewater, the spontaneous electrochemical method achieves 12-fold higher uranium extraction efficiency in comparison with the adsorption method. Using real wastewater, the uranium extraction efficiency reaches 303 mg g−1 upon 60 h operation with simultaneous net electrical energy production of 0.65 Wh m−2 with an operating cost of only USD 3.94–6.94 per kg of U. This work can pave a new avenue for cost-effective uranium recovery from mine wastewater.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data that support the findings of this study are presented in the article and Supplementary Information. Source data are provided with this paper. The data that support the findings of this study are openly available in the Figshare repository with identifier https://doi.org/10.6084/m9.figshare.23993085.
References
Rhodes, R. More nuclear power can speed CO2 cuts. Nature 548, 281 (2017).
Uranium 2020 (NEA, IAEA, 2021).
Liu, C. et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2, 17007 (2017).
Abney, C. W., Mayes, R. T., Saito, T. & Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 117, 13935–14013 (2017).
Tsouris, C. Uranium extraction: fuel from seawater. Nat. Energy 2, 17022 (2017).
Zheng, M. et al. Efficient adsorption of europium(III) and uranium(VI) by titanate nanorings: insights into radioactive metal species. Environ. Sci. Ecotechnol. 2, 100031 (2020).
Tsarev, S., Collins, R. N., Fahy, A. & Waite, T. D. Reduced uranium phases produced from anaerobic reaction with nanoscale zerovalent Iron. Environ. Sci. Technol. 50, 2595–2601 (2016).
Yuan, Y. et al. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat. Sustain. 4, 708–714 (2021).
Yuan, Y. et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angew. Chem. Int. Ed. 58, 11785–11790 (2019).
Yang, L. et al. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 5, 71–80 (2022).
Cui, W.-R. et al. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 11, 436 (2020).
Yuan, Y. et al. A bio-inspired nano-pocket spatial structure for targeting uranyl capture. Angew. Chem. Int. Ed. 59, 4262–4268 (2020).
Yang, H. et al. Functionalized iron–nitrogen–carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv. Mater. 33, 2106621 (2021).
Wang, Z. et al. Constructing an ion pathway for uranium extraction from seawater. Chem 6, 1683–1691 (2020).
Chi, F., Zhang, S., Wen, J., Xiong, J. & Hu, S. Highly efficient recovery of uranium from seawater using an electrochemical approach. Ind. Eng. Chem. Res. 57, 8078–8084 (2018).
Gu, B., Liang, L., Dickey, M. J., Yin, X. & Dai, S. Reductive precipitation of uranium(VI) by zero-valent iron. Environ. Sci. Technol. 32, 3366–3373 (1998).
Fiedor, J. N., Bostick, W. D., Jarabek, R. J. & Farrell, J. Understanding the mechanism of uranium removal from groundwater by zero-valent iron using X-ray photoelectron spectroscopy. Environ. Sci. Technol. 32, 1466–1473 (1998).
Zhao, X. et al. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 100, 245–266 (2016).
Ruan, Y. et al. Phosphate enhanced uranium stable immobilization on biochar supported nano zero valent iron. J. Hazard. Mater. 424, 127119 (2022).
Zhang, X. et al. Controllable shell corrosion of coated nanoscale zero valent iron induces long-term potentiation of its reactivity for uranium removal. Sep. Purif. Technol. 287, 120550 (2022).
Ling, L. & Zhang, W.-X. Enrichment and encapsulation of uranium with iron nanoparticle. J. Am. Chem. Soc. 137, 2788–2791 (2015).
Tsarev, S., Collins, R. N., Ilton, E. S., Fahy, A. & Waite, T. D. The short-term reduction of uranium by nanoscale zero-valent iron (nZVI): role of oxide shell, reduction mechanism and the formation of U(V)-carbonate phases. Environ. Sci. Nano 4, 1304–1313 (2017).
Hua, Y., Wang, W., Hu, N., Gu, T., Ling, L., & Zhang, W.-x. Enrichment of uranium from wastewater with nanoscale zero-valent iron (nZVI). Environ. Sci. Nano 8, 666–674 (2021).
Bae, S., Collins, R. N., Waite, T. D. & Hanna, K. Advances in surface passivation of nanoscale zerovalent iron: a critical review. Environ. Sci. Technol. 52, 12010–12025 (2018).
Mortazavian, S., An, H., Chun, D. & Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: material characterizations and kinetic studies. Chem. Eng. J. 353, 781–795 (2018).
Guan, X. et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 75, 224–248 (2015).
Sun, M. et al. Electrochemical–osmotic process for simultaneous recovery of electric energy, water, and metals from wastewater. Environ. Sci. Technol. 54, 8430–8442 (2020).
Carvalho, F. & Oliveira, J. Alpha emitters from uranium mining in the environment. J. Radioanal. Nucl. Chem. 274, 167–174 (2007).
Li, P. et al. Uranium elimination and recovery from wastewater with ligand chelation-enhanced electrocoagulation. Chem. Eng. J. 393, 124819 (2020).
Yang, P., Li, S., Liu, C., Shen, C. & Liu, X. Water-endurable intercalated graphene oxide adsorbent with highly efficient uranium capture from acidic wastewater. Sep. Purif. Technol. 263, 118364 (2021).
Cheng, Y. et al. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 367, 198–207 (2019).
Li, J. et al. Removal of uranium from uranium plant wastewater using zero-valent iron in an ultrasonic field. Nucl. Eng. Technol. 48, 744–750 (2016).
Wang, J. et al. Surface water contamination by uranium mining/milling activities in Northern Guangdong Province, China. CLEAN – Soil, Air, Water 40, 1357–1363 (2012).
Liu, T. et al. Removal and recovery of uranium from groundwater using direct electrochemical reduction method: performance and implications. Environ. Sci. Technol. 53, 14612–14619 (2019).
Yuan, K., Renock, D., Ewing, R. C. & Becker, U. Uranium reduction on magnetite: probing for pentavalent uranium using electrochemical methods. Geochim. Cosmochim. Acta 156, 194–206 (2015).
Yuan, K. et al. Electrochemical and spectroscopic evidence on the one-electron reduction of U(VI) to U(V) on magnetite. Environ. Sci. Technol. 49, 6206–6213 (2015).
Wen, Y. et al. Electrochemical reactors for continuous decentralized H2O2 production. Angew. Chem. Int. Ed. 61, e202205972 (2022).
Corbel, C. et al. Addition versus radiolytic production effects of hydrogen peroxide on aqueous corrosion of UO2. J. Nucl. Mater. 348, 1–17 (2006).
Guo, X. et al. Energetics of metastudtite and implications for nuclear waste alteration. Proc. Natl Acad. Sci. USA 111, 17737–17742 (2014).
Kubatko, K.-A. H., Helean, K. B., Navrotsky, A. & Burns, P. C. Stability of peroxide-containing uranyl minerals. Science 302, 1191–1193 (2003).
Li, X. et al. High valence Mo-doped Na3V2(PO4)3/C as a high rate and stable cycle-life cathode for sodium battery. J. Mater. Chem. A 6, 1390–1396 (2018).
Li, Q. et al. Silica restricting the sulfur volatilization of nickel sulfide for high-performance lithium-ion batteries. Adv. Energy Mater. 9, 1901153 (2019).
Zahiri, B. et al. Revealing the role of the cathode–electrolyte interface on solid-state batteries. Nat. Mater. 20, 1392–1400 (2021).
Boyle, D. T. et al. Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat. Energy 6, 487–494 (2021).
Ye, Y. et al. Efficient and durable uranium extraction from uranium mine tailings seepage water via a photoelectrochemical method. iScience 24, 103230 (2021).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Weck, P. F., Kim, E., Jové-Colón, C. F. & Sassani, D. C. Structures of uranyl peroxide hydrates: a first-principles study of studtite and metastudtite. Dalton Trans. 41, 9748–9752 (2012).
Walshe, A., Prüßmann, T., Vitova, T. & Baker, R. J. An EXAFS and HR-XANES study of the uranyl peroxides [UO2(η2-O2)(H2O)2]·nH2O (n = 0, 2) and uranyl (oxy)hydroxide [(UO2)4O(OH)6]·6H2O. Dalton Trans. 43, 4400–4407 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 42077352 to Y.W.) and the Fundamental Research Funds for the Central Universities (no. 31020200QD024 to Y.Y., no. 3102019JC007 to Y.W. and no. G2021KY0601 to Y.W.).
Author information
Authors and Affiliations
Contributions
Y.Y., F.C. and J.J. designed the research; Y.Y. and J.J. conducted the formal analysis; Y.Y., F.C. and Y.W. acquired the funding for the research; J.J., W.H., X.T., Z.Q., Y.C., C.L, Y.F. and S.M. performed the investigation; Y.Y., F.C. and J.J. developed the methodology; Y.Y., F.C. and Y.W. managed the project. Y.Y., F.C., S.M. and Y.W. provided the resources; Y.Y., F.C. and Y.W. supervised the research; Y.Y., F.C. and Y.W. validated the findings; Y.Y. visualized the data; Y.Y. drafted the manuscript; and F.C., Y.W. and Y.F. wrote, reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks He Tian, Beniamin Zahiri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Tables 1–3 and Note 1.
Supplementary Video 1
Morphological change of the CCF electrode surface during SPEC uranium extraction.
Supplementary Video 2
Simultaneous electricity output during SPEC uranium extraction.
Source data
Source Data Fig. 1
Numeric source data.
Source Data Fig. 2
Numeric source data.
Source Data Fig. 3.
Numeric source data.
Source Data Fig. 4
Numeric source data.
Source Data Fig. 6
Numeric source data.
Source Data Fig. 7
Numeric source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Y., Jin, J., Han, W. et al. Spontaneous electrochemical uranium extraction from wastewater with net electrical energy production. Nat Water 1, 887–898 (2023). https://doi.org/10.1038/s44221-023-00134-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-023-00134-0