Abstract
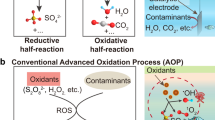
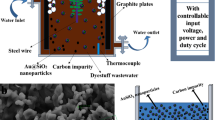
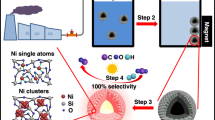
The presence of organic contaminants in wastewater poses considerable risks to the health of both humans and ecosystems. Although advanced oxidation processes that rely on highly reactive radicals to destroy organic contaminants are appealing treatment options, substantial energy and chemical inputs limit their practical applications. Here we demonstrate that Cu single atoms incorporated in graphitic carbon nitride can catalytically activate H2O2 to generate hydroxyl radicals at pH 7.0 without energy input, and show robust stability within a filtration device. We further design an electrolysis reactor for the on-site generation of H2O2 from air, water and renewable energy. Coupling the single-atom catalytic filter and the H2O2 electrolytic generator in tandem delivers a wastewater treatment system. These findings provide a promising path toward reducing the energy and chemical demands of advanced oxidation processes, as well as enabling their implementation in remote areas and isolated communities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Miklos, D. B. et al. Evaluation of advanced oxidation processes for water and wastewater treatment—a critical review. Water Res. 139, 118–131 (2018).
Chuang, Y.-H., Chen, S., Chinn, C. J. & Mitch, W. A. Comparing the UV/monochloramine and UV/free chlorine advanced oxidation processes (AOPs) to the UV/hydrogen peroxide AOP under scenarios relevant to potable reuse. Environ. Sci. Technol. 51, 13859–13868 (2017).
Hodges, B. C., Cates, E. L. & Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Glaze, W. H., Kang, J.-W. & Chapin, D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 9, 335–352 (1987).
Katsoyiannis, I. A., Canonica, S. & von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 45, 3811–3822 (2011).
Neyens, E. & Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 98, 33–50 (2003).
Nidheesh, P. V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. RSC Adv. 5, 40552–40577 (2015).
Pham, A. L.-T., Lee, C., Doyle, F. M. & Sedlak, D. L. A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 43, 8930–8935 (2009).
Lyu, L., Zhang, L., Wang, Q., Nie, Y. & Hu, C. Enhanced Fenton catalytic efficiency of γ-Cu–Al2O3 by σ-Cu2+–ligand complexes from aromatic pollutant degradation. Environ. Sci. Technol. 49, 8639–8647 (2015).
Costa, R. C. C. et al. Novel active heterogeneous Fenton system based on Fe3-xMxO4 (Fe, Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J. Hazard. Mater. 129, 171–178 (2006).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
Navalon, S., Alvaro, M. & Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B 99, 1–26 (2010).
Navalon, S., Dhakshinamoorthy, A., Alvaro, M. & Garcia, H. Heterogeneous fenton catalysts based on activated carbon and related materials. ChemSusChem 4, 1712–1730 (2011).
Bataineh, H., Pestovsky, O. & Bakac, A. pH-induced mechanistic changeover from hydroxyl radicals to iron(IV) in the Fenton reaction. Chem. Sci. 3, 1594–1599 (2012).
Lin, S.-S. & Gurol, M. D. Catalytic decomposition of hydrogen peroxide on iron oxide: kinetics, mechanism, and implications. Environ. Sci. Technol. 32, 1417–1423 (1998).
Campos-Martin, J. M., Blanco-Brieva, G. & Fierro, J. L. G. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. Engl. 45, 6962–6984 (2006).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Kim, H. W. et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 1, 282–290 (2018).
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Choi, C. H. et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 7, 10922 (2016).
Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Chen, Z. et al. Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. React. Chem. Eng. 2, 239–245 (2017).
Murayama, T. & Yamanaka, I. Electrosynthesis of neutral H2O2 solution from O2 and water at a mixed carbon cathode using an exposed solid-polymer-electrolyte electrolysis cell. J. Phys. Chem. C. 115, 5792–5799 (2011).
Yamanaka, I. & Murayama, T. Neutral H2O2 synthesis by electrolysis of water and O2. Angew. Chem. Int. Ed. Engl. 47, 1900–1902 (2008).
Bojdys, M. J., Müller, J.-O., Antonietti, M. & Thomas, A. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chemistry 14, 8177–8182 (2008).
Liu, J., Zhang, T., Wang, Z., Dawson, G. & Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 21, 14398–14401 (2011).
Natarajan, T. S., Thomas, M., Natarajan, K., Bajaj, H. C. & Tayade, R. J. Study on UV-LED/TiO2 process for degradation of rhodamine B dye. Chem. Eng. J. 169, 126–134 (2011).
He, Z. et al. Photocatalytic degradation of rhodamine B by Bi2WO6 with electron accepting agent under microwave irradiation: mechanism and pathway. J. Hazard. Mater. 162, 1477–1486 (2009).
Fu, H., Pan, C., Yao, W. & Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 109, 22432–22439 (2005).
Yamanaka, K. Anodically electrodeposited iridium oxide films (AEIROF) from alkaline solutions for electrochromic display devices. Jpn. J. Appl. Phys. 28, 632 (1989).
Feng, D. et al. Zirconium-metalloporphyrin PCN-222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. Engl. 51, 10307–10310 (2012).
Acknowledgements
Part of this work was performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation under award no. ECCS-1542152. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. The teratogenicity experiment was supported by NIH grant no. R35 GM127030.
Author information
Authors and Affiliations
Contributions
J.X. and Y.C. conceived the idea. J.X. performed the experiments. X.Z. performed the EXAFS and STEM characterizations. Z.F. performed the teratogenicity studies. Z.L. synthesized the O-SP. W.H., Y.L. and Z.Z. performed the HR-TEM and EDS characterizations. D.V. and Y.L. helped with the HPLC and LC–MS measurements. S.D. helped with the STEM characterizations. K.W. synthesized Cu-TMCPP. Z.L. and G.C. helped with quantification of H2O2. H.W. and Z.Z. helped with electrochemistry experiments. J.X. and Y.C. wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Discussion.
Supplementary Video
Filtration process of the Fenton filter.
Rights and permissions
About this article
Cite this article
Xu, J., Zheng, X., Feng, Z. et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat Sustain 4, 233–241 (2021). https://doi.org/10.1038/s41893-020-00635-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-00635-w
This article is cited by
-
Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application
Nature Communications (2024)
-
Wood-inspired metamaterial catalyst for robust and high-throughput water purification
Nature Communications (2024)
-
Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water
Nature Communications (2024)
-
Combined advanced oxidation dye-wastewater treatment plant: design and development with data-driven predictive performance modeling
npj Clean Water (2024)
-
Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production
Nature Communications (2024)