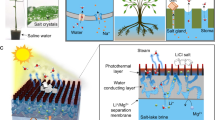
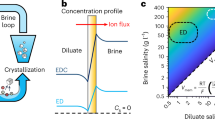
Abstract
Limited lithium supply is hindering the global transformation towards electrification and decarbonization. Current lithium mining can be energy, chemical and land intensive. Here we present an efficient and self-concentrating crystallization method for the selective extraction of lithium from both brine and seawater. The sequential and separable crystallization of cation species with different concentrations and solubilities was enabled by a twisted and slender 3D porous natural cellulose fibre structure via capillary and evaporative flows. The process exhibited an evaporation rate as high as 9.8 kg m−2 h−1, and it selectively concentrated lithium by orders of magnitude. The composition and spatial distribution of crystals were characterized, and a transport model deciphered the ion re-distribution process in situ. We also demonstrated system scalability via a 100-crystallizer array.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are presented in the article and its Supplementary Information. Source data are provided with this paper.
References
Goldthau, A. & Hughes, L. Protect global supply chains for low-carbon technologies. Nature 585, 28–30 (2020).
Greim, P., Solomon, A. A. & Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 11, 1–11 (2020).
Sun, X., Liu, Z., Zhao, F. & Hao, H. Global competition in the lithium-ion battery supply chain: a novel perspective for criticality analysis. Environ. Sci. Technol. 55, 12180–12190 (2021).
Raw materials for a truly green future. Nat. Rev. Mater. 6, 455 (2021).
Miatto, A., Wolfram, P., Reck, B. K. & Graedel, T. E. Uncertain future of American lithium: a perspective until 2050. Environ. Sci. Technol. 55, 16184–16194 (2021).
Lithium. TRADING ECONOMICS https://tradingeconomics.com/commodity/lithium (2023).
Azevedo, M., Baczynska, M., Hoffman, K. & Krauze, A. Lithium mining: how new production technologies could fuel the global EV revolution. McKinsey & Company https://www.mckinsey.com/industries/metals-and-mining/our-insights/lithium-mining-how-new-production-technologies-could-fuel-the-global-ev-revolution (2022).
Jaskula, B.W. USGS: 2015 Minerals Yearbook: lithium [advanced released]. https://minerals.usgs.gov/minerals/pubs/commodity/lithium/myb1-2015-lithi.pdf (2017).
Yao, S. Lithium costs up in 2021, continuing to surge in 2022. S&P Global Market Intelligence. https://www.spglobal.com/marketintelligence/en/news-insights/research/lithium-costs-up-in-2021-continuing-to-surge-in-2022 (2022).
Murodjon, S., Yu, X., Li, M., Duo, J. & Deng, T. Lithium recovery from brines including seawater, salt lake brine, underground water and geothermal water. Thermodyn. Energy Eng. https://doi.org/10.5772/intechopen.90371 (2020).
He, X., Kaur, S. & Kostecki, R. Mining lithium from seawater. Joule 4, 1357–1358 (2020).
Grosjean, C., Herrera Miranda, P., Perrin, M. & Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 16, 1735–1744 (2012).
Yang, S., Zhang, F., Ding, H., He, P. & Zhou, H. Lithium metal extraction from seawater. Joule 2, 1648–1651 (2018).
Xu, J. et al. A green and sustainable strategy toward lithium resources recycling from spent batteries. Sci. Adv. 8, eabq7948 (2022).
Lithium Market—Industry Analysis and Forecast (2022–2029) (Maximize Market Research Pvt, 2019).
Sociedad Química y Minera de Chile S.A. (SQM 2021): Annual Reports https://ir.sqm.com/English/financials/annual-reports/default.aspx (2021).
Gutiérrez, J. S., Navedo, J. G. & Soriano-Redondo, A. Atacama imperilled by lithium mining. Nature https://doi.org/10.1038/d41586-018-05233-7 (2018).
Lèbre, É. et al. The social and environmental complexities of extracting energy transition metals. Nat. Commun. https://doi.org/10.1038/s41467-020-18661-9 (2020).
Amoatey, P. et al. A critical review of environmental and public health impacts from the activities of evaporation ponds. Sci. Total Environ. 796, 149065 (2021).
Safari, S., Lottermoser, B. G. & Alessi, D. S. Metal oxide sorbents for the sustainable recovery of lithium from unconventional resources. Appl. Mater. Today 19, 100638 (2020).
Stringfellow, W. T. & Dobson, P. F. Technology for lithium extraction in the context of hybrid geothermal power. Proc. 46th Work. Geotherm. Reserv. Eng. 46, 1–20 (2021).
Li, X. et al. Membrane-based technologies for lithium recovery from water lithium resources: a review. J. Memb. Sci. 591, 117317 (2019).
Liu, G., Zhao, Z. & Ghahreman, A. Novel approaches for lithium extraction from salt-lake brines: a review. Hydrometallurgy 187, 81–100 (2019).
Sun, Y., Wang, Q., Wang, Y., Yun, R. & Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 256, 117807 (2021).
Zhao, X., Yang, H., Wang, Y. & Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 850, 113389 (2019).
Parsa, N., Moheb, A., Mehrabani-Zeinabad, A. & Masigol, M. A. Recovery of Lithium Ions from Sodium-Contaminated Lithium Bromide Solution by Using Electrodialysis Process. Chemical Engineering Research and Design vol. 98 (Institution of Chemical Engineers, 2015).
Hoshino, T. Preliminary studies of lithium recovery technology from seawater by electrodialysis using ionic liquid membrane. Desalination 317, 11–16 (2013).
Warnock, S. J. et al. Engineering Li/Na selectivity in 12-Crown-4-functionalized polymer membranes. Proc. Natl Acad. Sci. USA 118, 1–8 (2021).
Habata, Y., Ikeda, M. & Akabori, S. Lithium ion selective dibenzo-14-crown-4 possessing a phosphoric acid functional group as a pendant. Tetrahedron Lett. 33, 3157–3160 (1992).
Hano, T., Matsumoto, M., Ohtake, T., Egashira, N. & Hori, F. Recovery of lithium from geothermal water by solvent extraction technique. Solvent Extr. Ion Exch. 10, 195–206 (1992).
Swain, B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: a review. J. Chem. Technol. Biotechnol. 91, 2549–2562 (2016).
Yim, C.-H. & Abu-Lebdeh, Y. A. Connection between phase diagram, structure and ion transport in liquid, aqueous electrolyte solutions of lithium chloride. J. Electrochem. Soc. 165, A547–A556 (2018).
Report, O. & Atacama, S. D. E. Technical Report Summary Operation Report Salar de Atacama (2022).
Kuznetsov, G. V. et al. Evaporation modes of LiBr, CaCl2, LiCl, NaCl aqueous salt solution droplets on aluminum surface. Int. J. Heat Mass Transf. 126, 161–168 (2018).
Lu, L. et al. Unbiased solar H2 production with current density up to 23 mA cm−2 by Swiss-cheese black Si coupled with wastewater bioanode. Energy Environ. Sci. 12, 1088–1099 (2019).
Chen, X. et al. Sustainable off-grid desalination of hypersaline waters using Janus wood evaporators. Energy Environ. Sci. 14, 5347–5357 (2021).
Zheng, S. et al. Upscaling 3D engineered trees for off-grid desalination. Environ. Sci. Technol. 56, 1289–1299 (2022).
Jiang, J., Chen, X., Chen, X. & Ren, Z. J. Energy-efficient microbial electrochemical lignin and alkaline hydroxide recovery from DMR black liquor. Resour. Conserv. Recycl. 186, 106529 (2022).
Guglielmini, L., Gontcharov, A., Aldykiewicz, A. J. & Stone, H. A. Drying of salt solutions in porous materials: intermediate-time dynamics and efflorescence. Phys. Fluids 20, 077101 (2008).
Li, C. et al. Mapping techniques for the design of lithium-sulfur batteries. Small 18, 1–14 (2022).
Adhitama, E. et al. Pre‐lithiation of silicon anodes by thermal evaporation of lithium for boosting the energy density of lithium ion cells. Adv. Funct. Mater. 32, 2201455 (2022).
Acknowledgements
The authors appreciate the support from Princeton Catalysis Initiative (PCI), and we acknowledge the use of Princeton’s Imaging and Analysis Center, which is partially supported through the Princeton Center for Complex Materials (PCCM), a National Science Foundation (NSF)-MRSEC programme (DMR-2011750). F.T.-C. and S.Z. gratefully acknowledge the Distinguished Postdoctoral Fellowships from the Andlinger Center for Energy and the Environment.
Author information
Authors and Affiliations
Contributions
X.C. conceived the initial idea with the input from group members. X.C., M.Y., S.Z., Z.J.R. and L.H. contributed to the experimental design. Z.J.R. supervised the study. X.C., M.Y. and S.Z. conducted materials preparation and system operation. F.T.-C. and H.A.S. carried out model development. X.C., G.C. and N.Y. conducted material characterization. X.C., M.Y. and S.Z. contributed to experimental analysis. X.C. and Q.D. contributed to schematics design. X.C., M.Y., F.T.-C. and Z.J.R. wrote the paper, and all authors commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
X.C., S.Z. and Z.R. are authors on a patent application (PCT/US22/50915) for fibre evaporators. The other authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Ping He and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4 and Figs. 1–20, and captions for Supplementary Movies 1–3.
Supplementary Movie 1
Easy peeling of the Na-rich salt shell for Na separation, sampled after 5 days’ spatial crystallization for Li extraction from seawater.
Supplementary Movie 2
3D structure of the four-strings-twisted fibre crystallizer. Movie constructed by AVIZO software upon 3D X-ray scanning images.
Supplementary Movie 3
Time lapse video records the growth of the crystals during the operation. From left to right, the saline water contains 10, 40, 70 and 100 g l−1 NaCl, respectively. Visible salt crystalized at different heights, with the lower concentration crystalized at a higher position.
Supplementary Data 1
Data of supplementary figures.
Source data
Source Data Fig. 1
Statistical source data of Fig. 1.
Source Data Fig. 4
Statistical source data of Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Yang, M., Zheng, S. et al. Spatially separated crystallization for selective lithium extraction from saline water. Nat Water 1, 808–817 (2023). https://doi.org/10.1038/s44221-023-00131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-023-00131-3
This article is cited by
-
A fast evaporative method for extracting lithium from brines
Nature Water (2023)