Abstract
The complexity of neural activity is a commonly used readout of healthy functioning in cortical circuits. Previous work has linked neural complexity to the level of maternal care in preterm infants at risk for developing mental disorders, yet the evolution of neural complexity in early human development is largely unknown. We hypothesized that cortical dynamics would evolve to optimize information processing as birth approaches, thereby increasing the complexity of cortical activity. To test this hypothesis, we conducted a cohort study relating prenatal neural complexity to maturation. Magnetoencephalography (MEG) recordings were obtained from a sample of fetuses and newborns, including longitudinal data before and after birth. Using cortical responses to auditory irregularities, we computed several entropy measures that reflect the complexity of the MEG signal. Despite our hypothesis, neural complexity decreased significantly with maturation in both fetuses and newborns. Furthermore, we found that complexity decreased significantly faster in male fetuses for most entropy measures. Our surprising results chart the evolution of neural complexity in perinatal human development and may lay a foundation for future work that would relate fetal neural complexity to developmental phenotypes, especially in the area of perinatal risk where biomarkers are greatly needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data used in this study are already publicly archived and available through Zenodo88,89. Fetal data were originally published in ref. 26 and are available at https://zenodo.org/record/4541463#.Y0a-iExByHt. Neonatal data were originally published in ref. 25 and are available at https://zenodo.org/record/4018827#.Y0a-akxByHt.
Code availability
Analysis code is publicly available at https://github.com/jfrohlich/Fetal_MEG_Entropy/. Other code relevant to the data preprocessing is publicly available at https://github.com/moser297/fMEG_data_processing/tree/main.
References
Bosl, W. J., Tager-Flusberg, H. & Nelson, C. A. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci. Rep. 8, 6828 (2018).
Guan, S. et al. The complexity of spontaneous brain activity changes in schizophrenia, bipolar disorder and ADHD was examined using different variations of entropy. Hum. Brain Mapp. 44, 94–118 (2023).
Ab´asolo, D. et al. Analysis of regularity in the EEG background activity of Alzheimer’s disease patients with approximate entropy. Clin. Neurophysiol. 116, 1826–1834 (2005).
Sun, J. et al. Complexity analysis of EEG, MEG and fMRI in mild cognitive impairment and Alzheimer’s disease: a review. Entropy 22, 239 (2020).
Casali, A. G. et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 5, 198ra105 (2013).
Sarasso, S. et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon and ketamine. Curr. Biol. 25, 3099–3105 (2015).
Mediano, P. A. et al. Fluctuations in neural complexity during wakefulness relate to conscious level and cognition. Preprint at https://www.biorxiv.org/content/10.1101/2021.09.23.461002v1 (2021).
Timmermann, C. et al. Human brain effects of DMT assessed via EEG-fMRI. Proc. Natl Acad. Sci. USA 120, e2218949120 (2023).
Schartner, M. M., Carhart-Harris, R. L., Barrett, A. B., Seth, A. K. & Muthukumaraswamy, S. D. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci. Rep. 7, 46421 (2017).
Toker, D. et al. Consciousness is supported by near-critical slow cortical electrodynamics. Proc. Natl Acad. Sci. USA 119, e2024455119 (2022).
Ivanov, P. C. et al. Multifractality in human heartbeat dynamics. Nature 399, 461–465 (1999).
Takahashi, T. et al. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 51, 173–182 (2010).
Johnson, S. et al. Psychiatric disorders in extremely preterm children: longitudinal findings at age 11 years in the EPICure study. J. Am. Acad. Child Adolesc. Psychiatry 49, 453–463 (2010).
Nosarti, C. et al. Preterm birth and psychiatric disorders in young adult life. Arch. General Psychiatry 69, 610–617 (2012).
Janjarasjitt, S., Scher, M. & Loparo, K. Nonlinear dynamical analysis of the neonatal EEG time series: the relationship between sleep state and complexity. Clin. Neurophysiol. 119, 1812–1823 (2008).
Scher, M. S., Waisanen, H., Loparo, K. & Johnson, M. W. Prediction of neonatal state and maturational change using dimensional analysis. J. Clin. Neurophysiol. 22, 159–165 (2005).
Kaffashi, F., Scher, M., Ludington-Hoe, S. & Loparo, K. An analysis of the kangaroo care intervention using neonatal EEG complexity: a preliminary study. Clin. Neurophysiol. 124, 238–246 (2013).
Isler, J. R., Stark, R. I., Grieve, P. G., Welch, M. G. & Myers, M. M. Integrated information in the EEG of preterm infants increases with family nurture intervention, age and conscious state. PLoS ONE 13, e0206237 (2018).
De Wel, O. et al. Complexity analysis of neonatal EEG using multiscale entropy: applications in brain maturation and sleep stage classification. Entropy 19, 516 (2017).
Semeia, L. et al. Multiscale entropy analysis of combined eeg-fnirs measurement in preterm neonates. Preprint at https://www.biorxiv.org/content/10.1101/2023.07.12.548724v1 (2023).
Sortica da Costa, C. et al. Complexity of brain signals is associated with outcome in preterm infants. J. Cereb. Blood Flow Metab. 37, 3368–3379 (2017).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Frohlich, J. et al. Not with a ‘zap’ but with a ‘beep’: measuring the origins of perinatal experience: origins of perinatal experience. NeuroImage. 273, 120057 (2023).
Rajagopalan, V. et al. Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J. Neurosci. 31, 2878–2887 (2011).
Moser, J. et al. Magnetoencephalographic signatures of hierarchical rule learning in newborns. Dev. Cogn. Neurosci. 46, 100871 (2020).
Moser, J. et al. Magnetoencephalographic signatures of conscious processing before birth. Dev. Cogn. Neurosci. 49, 100964 (2021).
Morokuma, S. et al. Fetal habituation correlates with functional brain development. Behav. Brain Res. 153, 459–463 (2004).
Tononi, G., Boly, M., Massimini, M. & Koch, C. Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17, 450–461 (2016).
Toker, D., Sommer, F. T. & D’Esposito, M. A simple method for detecting chaos in nature. Commun. Biol. 3, 11 (2020).
Eswaran, H. et al. Tracking evoked responses to auditory and visual stimuli in fetuses exposed to maternal high-risk conditions. Dev. Psychobiol. 63, 5–15 (2021).
Moser, J. et al. Evaluating complexity of fetal MEG signals: a comparison of different metrics and their applicability. Front. Syst. Neurosci. 13, 23 (2019).
Semeia, L., Sippel, K., Moser, J. & Preissl, H. Evaluation of parameters for fetal behavioural state classification. Sci. Rep. 12, 3410 (2022).
Sippel, K. et al. Fully automated subtraction of heart activity for fetal magnetoencephalography data. In Proc. 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 5685–5689 (IEEE, 2019).
Lempel, A. & Ziv, J. On the complexity of finite sequences. IEEE Trans. Inf. Theory 22, 75–81 (1976).
Willems, F. M., Shtarkov, Y. M. & Tjalkens, T. J. The context-tree weighting method: basic properties. IEEE Trans. Inf. Theory 41, 653–664 (1995).
Xie, H. B., He, W. X. & Liu, H. Measuring time series regularity using nonlinear similarity-based sample entropy. Phys. Lett. A 372, 7140–7146 (2008).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 89, 068102 (2002).
Bandt, C. & Pompe, B. Permutation entropy: a natural complexity measure for time series. Phys. Rev. Lett. 88, 174102 (2002).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009).
Mensen, A. & Khatami, R. Advanced EEG analysis using threshold-free cluster-enhancement and non-parametric statistics. Neuroimage 67, 111–118 (2013).
Mediano, P. A., Rosas, F. E., Barrett, A. B. & Bor, D. Decomposing spectral and phasic differences in nonlinear features between datasets. Phys. Rev. Lett. 127, 124101 (2021).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Software 59, 1–38 (2014).
Gao, Y., Kontoyiannis, I. & Bienenstock, E. Estimating the entropy of binary time series: methodology, some theory and a simulation study. Entropy 10, 71–99 (2008).
Marshall, P. J., Bar-Haim, Y. & Fox, N. A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 113, 1199–1208 (2002).
Huo, J., Quan, S. F., Roveda, J. & Li, A. Coupling analysis of heart rate variability and cortical arousal using a deep learning algorithm. PLoS ONE 18, e0284167 (2023).
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. & Scammell, T. E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Edlow, A. G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagnosis 37, 95–110 (2017).
Cirulli, F., Musillo, C. & Berry, A. Maternal obesity as a risk factor for brain development and mental health in the offspring. Neuroscience 447, 122–135 (2020).
DiPietro, J. A. & Voegtline, K. M. The gestational foundation of sex differences in development and vulnerability. Neuroscience 342, 4–20 (2017).
Fombonne, E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J. Autism Dev. Disord. 33, 365–382 (2003).
Greven, C. U., Richards, J. S. & Buitelaar, J. K. in Oxford Textbook of Attention Deficit Hyperactivity Disorder (eds Banaschewski, T., Coghill, D. & Zuddas, A.) 154–160 (Oxford Univ. Press, 2018).
Hodes, G. E. & Kropp, D. R. Sex as a biological variable in stress and mood disorder research. Nat. Mental Health 1, 453–461 (2023).
Wheelock, M. D. et al. Sex differences in functional connectivity during fetal brain development. Dev. Cogn. Neurosci. 36, 100632 (2019).
Cook, K. M. et al. Robust sex differences in functional brain connectivity are present in utero. Cereb. Cortex 33, 2441–2454 (2023).
Frohlich, J. et al. Neural complexity is a common denominator of human consciousness across diverse regimes of cortical dynamics. Commun. Biol. 5, 1374 (2022).
Lippé, S., Kovacevic, N. & McIntosh, R. Differential maturation of brain signal complexity in the human auditory and visual system. Front. Hum. Neurosci. 3, 48 (2009).
McIntosh, A. R., Kovacevic, N. & Itier, R. J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 4, e1000106 (2008).
Misić, B., Mills, T., Taylor, M. J. & McIntosh, A. R. Brain noise is task dependent and region specific. J. Neurophysiol. 104, 2667–2676 (2010).
Elston, G. N., Oga, T. & Fujita, I. Spinogenesis and pruning scales across functional hierarchies. J. Neurosci. 29, 3271–3275 (2009).
Peck, C. et al. Prediction of autism spectrum disorder diagnosis using nonlinear measures of language-related EEG at 6 and 12 months. J. Neurodev. Disord. 13, 57 (2021).
Corvilain, P. et al. Extending the applicability of optically pumped magnetoencephalography toward early human life. Preprint at https://www.biorxiv.org/content/10.1101/2023.10.28.564455v1 (2023).
Thomason, M. E. et al. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev. Cogn. Neurosci. 11, 96–104 (2015).
Barnett, L., Muthukumaraswamy, S. D., Carhart-Harris, R. L. & Seth, A. K. Decreased directed functional connectivity in the psychedelic state. NeuroImage 209, 116462 (2020).
Gao, W. et al. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl Acad. Sci. USA 106, 6790–6795 (2009).
Doss, M. K. et al. The acute effects of the atypical dissociative hallucinogen salvinorin A on functional connectivity in the human brain. Sci. Rep. 10, 16392 (2020).
Carhart-Harris, R. L. et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl Acad. Sci. USA 113, 4853–4858 (2016).
Deroy, O. & Spence, C. Are we all born synaesthetic? Examining the neonatal synaesthesia hypothesis. Neurosci. Biobehav. Rev. 37, 1240–1253 (2013).
Marks, L. E. & Odgaard, E. C. in Synesthesia: Perspectives from Cognitive Neuroscience (eds Robertson, L. C. & Sagiv, N.) 214–236 (Oxford Univ. Press, 2005).
Blumberg, M. S. & Adolph, K. E. Protracted development of motor cortex constrains rich interpretations of infant cognition. Trends Cogn. Sci. 27, 233–245 (2023).
Shi, F., Salzwedel, A. P., Lin, W., Gilmore, J. H. & Gao, W. Functional brain parcellations of the infant brain and the associated developmental trends. Cereb. Cortex 28, 1358–1368 (2018).
Mortaheb, S. et al. Altered subjective experience after psilocybin intake associates with a dynamic pattern of hyperconnected functional connectivity. Preprint at https://www.biorxiv.org/content/10.1101/2023.09.18.558309v1 (2023).
Sylvester, C. M. et al. Network-specific selectivity of functional connections in the neonatal brain. Cereb. Cortex 33, 2200–2214 (2023).
Sydnor, V. J. et al. Neurodevelopment of the association cortices: patterns, mechanisms and implications for psychopathology. Neuron 109, 2820–2846 (2021).
Brauchli, C., Elmer, S., Rogenmoser, L., Burkhard, A. & Jäncke, L. Top–down signal transmission and global hyperconnectivity in auditory-visual synesthesia: evidence from a functional EEG resting-state study. Hum. Brain Mapp. 39, 522–531 (2018).
Luppi, A. I. et al. LSD alters dynamic integration and segregation in the human brain. NeuroImage 227, 117653 (2021).
Farnes, N., Juel, B. E., Nilsen, A. S., Romundstad, L. G. & Storm, J. F. Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine-induced psychedelic state in humans. PLoS One 15, e0242056 (2020).
Ort, A. et al. TMS-EEG and resting-state EEG applied to altered states of consciousness: oscillations, complexity and phenomenology. iScience 26, 106589 (2023).
Mediano, P. A. M. et al. Effects of external stimulation on Psychedelic State Neurodynamics. ACS Chem. Neurosci. https://doi.org/10.1021/acschemneuro.3c00289 (2024).
Tsolaki, A., Kosmidou, V., Hadjileontiadis, L., Kompatsiaris, I. Y. & Tsolaki, M. Brain source localization of MMN, P300 and N400: aging and gender differences. Brain Res. 1603, 32–49 (2015).
Sabeti, M., Katebi, S., Rastgar, K. & Azimifar, Z. A multi-resolution approach to localize neural sources of P300 event-related brain potential. Comput. Methods Programs Biomed. 133, 155–168 (2016).
Itzchak, E. B. & Zachor, D. A. Who benefits from early intervention in autism spectrum disorders? Res. Autism Spectr. Disord. 5, 345–350 (2011).
Rajpal, H. et al. Psychedelics and schizophrenia: distinct alterations to Bayesian inference. NeuroImage 263, 119624 (2022).
Jiujias, M., Kelley, E. & Hall, L. Restricted, repetitive behaviors in autism spectrum disorder and obsessive-compulsive disorder: a comparative review. Child Psychiatry Hum. Dev. 48, 944–959 (2017).
Mogadam, A. et al. Magnetoencephalographic (MEG) brain activity during a mental flexibility task suggests some shared neurobiology in children with neurodevelopmental disorders. J. Neurodev. Disord. 11, 19 (2019).
Carhart-Harris, R. et al. Canalization and plasticity in psychopathology. Neuropharmacology 226, 109398 (2022).
Schaworonkow, N. & Voytek, B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev. Cogn. Neurosci. 47, 100895 (2021).
Brookes, M. J. et al. Magnetoencephalography with optically pumped magnetometers (OPM-MEG): the next generation of functional neuroimaging. Trends Neurosci. 45, 621–634 (2022).
Moser, J. Data for ‘Magnetoencephalographic Signatures of Hierarchical Rule Learning in Newborns’ https://doi.org/10.5281/zenodo.4018827 (2020).
Moser, J. Data for ‘Magnetoencephalographic Signatures of Conscious Processing before Birth’ https://doi.org/10.5281/zenodo.4541463 (2021).
Jaiswal, A. et al. Comparison of beamformer implementations for MEG source localization. NeuroImage. 216, 116797 (2020).
Sippel, K. et al. Fully automated R-peak detection algorithm (FLORA) for fetal magnetoencephalographic data. Comput. Methods Programs Biomed. 173, 35–41 (2019).
Schleger, F. et al. Magnetoencephalographic signatures of numerosity discrimination in fetuses and neonates. Dev. Neuropsychol. 39, 316–329 (2014).
Moser, J., Sippel, K., Schleger, F. & Preißl, H. Automated detection of fetal brain signals with principal component analysis. In Proc. 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 6549–6552 (IEEE, 2019).
Husin, H. Mat et al. Maternal weight, weight gain and metabolism are associated with changes in fetal heart rate and variability. Obesity 28, 114–121 (2020).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 278, H2039–H2049 (2000).
Nikulin, V. V. & Brismar, T. Comment on ‘Multiscale entropy analysis of complex physiologic time series’. Phys. Rev. Lett. 92, 089803 (2004).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Bates, D. et al. Linear mixed-effects models using eigen and S4. R Package Version 1.1-23 (2013). https://CRAN.R-project.org/package=lme4
Lancaster, G., Iatsenko, D., Pidde, A., Ticcinelli, V. & Stefanovska, A. Surrogate data for hypothesis testing of physical systems. Phys. Rep. 748, 1–60 (2018).
Schreiber, T. & Schmitz, A. Improved surrogate data for nonlinearity tests. Phys. Rev. Lett. 77, 635–638 (1996).
Acknowledgements
We are grateful to all volunteers and families who participated in our research. We would also like to thank D. Toker for his input on methodological aspects of our study, A. DallaVecchia for her input on ‘sensory PCI’, C. Chu for useful comments on our work, J. F. Hipp for contributing analysis code, S. Ruch for assisting with a code review, and T. Bayne for many enlightening discussions on infant cognition. Additionally, we thank the fMEG team for their contributions, including F. Schleger (original study design) and M. Weiss (data collection). Finally, we gratefully acknowledge the following funders: the German Federal Ministry of Education and Research (BMBF) grants Somnia (13GW0294, A.G.), Enable (13GW0359, A.G.) and Bevares (13GW0570, A.G.), the European Union’s Joint Programme for Neurodegenerative Disease Research (EU-JPND 2022-130, A.G.) grant Recast (01ED2309, A.G.) the FET Open Luminous project (H2020 FETOPEN-2014-2015-RIA under agreement no. 686764, H.P.) as part of the European Union’s Horizon 2020 research and 2014–2018 training program, the German Federal Ministry of Education and Research (BMBF) at the German Center for Diabetes Research (DZD01GI0925, H.P.), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; 493345456, J.M.) and the Wellcome Trust (grant no. 210920/Z/18/Z, P.M.).
Author information
Authors and Affiliations
Contributions
J.F., P.M., H.P. and A.G. were responsible for conceptualization of the current study’s hypotheses and analysis plan. J.M., H.P. and K.S. designed the experiments and acquired and preprocessed the data in the context of a prior study. J.F., J.M., K.S. and P.M. contributed code for data preprocessing and analysis. J.M. and K.S. performed data curation. J.F. performed the formal analysis, generated the display items, and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript. H.P. and A.G. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Chun Meng and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overview of the experiment.
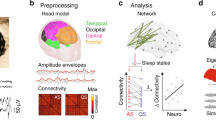
Musical notes denote auditory stimuli. (a) We used two block rules and the four possible permutations of local/global standard/deviant auditory sequences across both block rules. (b) Each auditory sequence consisted of four tones of 200 ms duration each, separated by a 400 ms inter-tone interval. The entire stimulus sequence was 2000 ms in duration. The fourth tone of each sequence varied during the test phase. After averaging across trials within each condition, we analyzed signals starting from 200 ms prior to the onset of the first tone to 1000 ms following the offset of the fourth tone (3200 ms duration). (c) For fetal recordings, the mother-to-be postioned her abdomen within the concavity of the MEG sensor array, with a sound balloon between her body and the SARA device delivering auditory tones. (d) Fetal MEG signals were recorded noninvasively in response to auditory tones. To correct for the influence of fetal head orientation and size on MEG signal amplitude, fetal signals were normalized. (e) After birth, a subset of subjects returned to the laboratory as newborns and were recorded from after being placed in a cradle oriented head-first toward the SARA device’s SQUID magnetometer array. Newborns wore infant-friendly headphones for stimulus delivery. (f) As with fetuses, the SARA device recorded cortical signals noninvasively from newborns. Note that photographs in c and e are adapted from ref. 23. The woman in c and the parents of the infant in e gave consent for identifiable images to be published.
Extended Data Fig. 2 Agreement between filtering.
High correlations between entropy measures computed from neonatal signals with narrow (1–10 Hz) versus broadband (1–15 Hz) filtering demonstrate that entropy is similar in both cases. We used 1–15 Hz filtering for the neonatal entropy analyzed in the manuscript.
Extended Data Fig. 3 Numbers of participants at each stage of the study, including recruitment, successful data collection, quality control, and followup visits to collect longitudinal data.
Numbers pertaining to fetal data are shown in red, whereas numbers pertaining to neonatal data are shown in blue. The Venn diagram at bottom right shows the total number of participants with usable data from before birth (N = 27), after birth (N = 4), and both before and after birth (N = 16). Note that all mothers-to-be who volunteered were found eligible to participate, thus an additional stage of confirmed eligibility is not depicted.
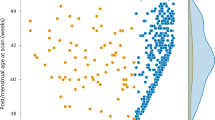
Extended Data Fig. 4 Scatter plots of postmenstrual age (PMA) versus neonatal MEG signal entropy.
Blue data points are taken from each session and rule/stimulus condition. For visualization purposes and R2 estimates, we took the median of data at the level of one-week time bins (magenta circles) and computed the variance explained R2 according to the least-squares fit of the smoothed data. For symmetry with Fig. 1 in the main manuscript, we plotted data separately for males (first and third columns) and females (second and fourth columns). The above data show that signal entropy generally declines with PMA in newborns. Note that some individual data points are excluded by the vertical axis scale due to the very large spread in entropy values.
Extended Data Fig. 5 Changes in entropy resulting from amplitude or non-amplitude signal changes.
Data distributions in each panel are averaged across all four experimental conditions (that is, block rule and stimulus combinations). PermEn32 and PermEn64 (middle row) showed a far larger dissociation between amplitude and non-amplitude signal properties, likely because the PermEn algorithm is sensitive to small changes in the signal which result in new ordinal rankings of data points. Effect sizes (Cohen’s d) are indicated for each decomposition.
Extended Data Fig. 6 Histograms of signal dynamics categories (stochastic or deterministic) by gestational age (fetuses, left column) and age (newborns, right column).
The first row shows results from global standards (a,b) and the second row shows results from global deviants (c,d). Both fetuses and newborns displayed a mixture of stochastic and deterministic dynamics. In fetuses, the majority of recordings were deterministic, whereas in newborns, the majority of recordings were stochastic. Dynamics were not significantly predicted by maturation in either group, though the proportion of recordings with stochastic dynamics was significantly higher in newborns than in fetuses (two-tailed chi-squared test using all data from both global standards and deviants, χ2 = 28.6, P = 9.1 × 10−8).
Extended Data Fig. 7 Correlations between MEG measures.
Entropy measures were highly correlated with one another in both fetuses (a) and newborns (b). These same entropy measures show negative correlations with spectral power at most frequencies in fetuses (c) and all frequencies in newborns (d). Subsecond CTW did not correlate strongly with subsecond spectral power after averaging across conditions in fetuses (e) or newborns (f).
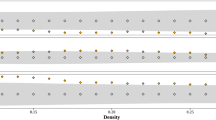
Extended Data Fig. 8 Alternative calculations of correlations in fetal entropy measures.
Because our fetal data contained multiple recordings from the same fetal subjects and, moreover, the random effect term significantly increased the fits of most models predicting entropy, we did not wish to rely on Pearson correlation coefficients (a) between entropy measures from each recording, as these correlation estimates may over-represent subjects with multiple recordings. For this reason, we instead utilized standardized model coefficients (betas) from linear mixed models that predicted entropy measures from each other while accounting for random effects (b). Differences between Pearson coefficients and averaged beta coefficients (c) are very small (β − r < 0.1 in all cases). However, standardized betas in LMMs are not generally symmetrical (that is, βi,j ≠ βj,i), since they depend on the variance of the random effect, and the random effect may often contribute more to one variable or the other. To address this problem, we used the mean of βi,j and βj,i to represent the correlation between entropy measure i and j. Here, for transparency, we show the asymmetry in the standardized betas prior to averaging (d), which primarily affected correlation estimates for PermEn64.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frohlich, J., Moser, J., Sippel, K. et al. Sex differences in prenatal development of neural complexity in the human brain. Nat. Mental Health 2, 401–416 (2024). https://doi.org/10.1038/s44220-024-00206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-024-00206-4
This article is cited by
-
KI-Visionen oder kybernetische Perspektiven?
Informatik Spektrum (2024)