Abstract
Ketamine may have antidepressant properties, but its acute psychoactive effects complicate successful masking in placebo-controlled trials. Here we present a single-center, parallel-arm, triple-masked, randomized, placebo-controlled trial assessing the antidepressant efficacy of intravenous ketamine masked by surgical anesthesia (ClinicalTrials.gov, NCT03861988). Adult patients (N = 40) with major depressive disorder who were scheduled for routine surgery were randomized to a single infusion of ketamine (0.5 mg kg−1) or placebo (saline) during usual anesthesia. All participants, investigators and direct-patient-care staff were masked to treatment allocation. The primary outcome was depression severity measured by the Montgomery–Åsberg Depression Rating Scale at 1, 2 and 3 days post-infusion. After all follow-up visits, participants were asked to guess which intervention they received. A mixed-effects model showed no evidence of effect of treatment assignment on the primary outcome (−5.82, 95% confidence interval −13.3 to 1.64, P = 0.13). Of all participants, 36.8% guessed their treatment assignment correctly; both groups allocated their guesses in similar proportions. In conclusion, a single dose of intravenous ketamine delivered during surgical anesthesia had no greater effect than placebo in acutely reducing the severity of depressive symptoms in adults with major depressive disorder. This trial successfully masked treatment allocation in patients with moderate-to-severe depression using surgical anesthesia. Although this masking strategy is impractical for most placebo-controlled trials, future studies of novel antidepressants with acute psychoactive effects should make efforts to fully mask treatment assignment to minimize participant-expectancy bias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
De-identified participant data, data dictionaries, the study protocol and the statistical analysis plan are available at https://osf.io/zdkr8/ (https://doi.org/10.17605/OSF.IO/ZDKR8). All participants have consented to sharing de-identified data with outside entities for scientific research purposes.
Code availability
R code used for data analysis is publicly available at https://osf.io/zdkr8/ (https://doi.org/10.17605/OSF.IO/ZDKR8).
References
Sleigh, J., Harvey, M., Voss, L. & Denny, B. Ketamine—more mechanisms of action than just NMDA blockade. Trends Anaesth. Crit. Care 4, 76–81 (2014).
Bonaventura, J. et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol. Psychiatry 26, 6704–6722 (2021).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000).
McIntyre, R. S. et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am. J. Psychiatry 178, 383–399 (2021).
Zarate, C. A. et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006).
Marcantoni, W. S. et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J. Affect. Disord. 277, 831–841 (2020).
Butler, M., Jelen, L. & Rucker, J. Expectancy in placebo-controlled trials of psychedelics: if so, so what? Psychopharmacology 239, 3047–3055 (2022).
Hall, W. D. & Humphreys, K. Is good science leading the way in the therapeutic use of psychedelic drugs? Psychol. Med. 52, 2849–2851 (2022).
Aday, J. S. et al. Great expectations: recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacology 239, 1989–2010 (2022).
Muthukumaraswamy, S. D., Forsyth, A. & Lumley, T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev. Clin. Pharmacol. 14, 1133–1152 (2021).
Price, R. B. et al. A novel, brief, fully automated intervention to extend the antidepressant effect of a single ketamine infusion: a randomized clinical trial. Am. J. Psychiatry 179, 959–968 (2022).
Horvath, B., Kloesel, B., Todd, M. M., Cole, D. J. & Prielipp, R. C. The evolution, current value, and future of the American Society of Anesthesiologists physical status classification system. Anesthesiology 135, 904–919 (2021).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987).
Nagele, P. et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci. Transl. Med. 13, eabe1376 (2021).
Phillips, J. L. et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am. J. Psychiatry 176, 401–409 (2019).
Murrough, J. W. et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142 (2013).
Sos, P. et al. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol. Lett. 34, 287–293. (2013).
Singh, J. B. et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am. J. Psychiatry 173, 816–826 (2016).
Sanacora, G. et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74, 399 (2017).
Dore, J. et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J. Psychoactive Drugs 51, 189–198 (2019).
Joneborg, I. et al. Active mechanisms of ketamine-assisted psychotherapy: a systematic review. J. Affect. Disord. 315, 105–112 (2022).
Nagele, P. et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol. Psychiatry 78, 10–18 (2015).
Mickey, B. J. et al. Propofol for treatment-resistant depression: a pilot study. Int. J. Neuropsychopharmacol. 21, 1079–1089 (2018).
Weeks, H. R. et al. Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. PLoS ONE 8, e69809 (2013).
García-Toro, M. et al. Inefficacy of burst-suppression anesthesia in medication-resistant major depression: a controlled trial. J. ECT 17, 284–288 (2001).
Kudoh, A., Takahira, Y., Katagai, H. & Takazawa, T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth. Analg. 95, 114–118 (2002).
Mashour, G. A. et al. Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Br. J. Anaesth. 121, 1075–1083 (2018).
Liu, P. et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J. Invest. Surg. 34, 883–888 (2021).
Zhou, Y. et al. Ketamine alleviates depressive symptoms in patients undergoing intracranial tumor resection: a randomized controlled trial. Anesth. Analg. 133, 1588–1597 (2021).
Guo, J. et al. Efficacy and safety of perioperative application of ketamine on postoperative depression: a meta-analysis of randomized controlled studies. Mol. Psychiatry https://doi.org/10.1038/s41380-023-01945-z (2023).
Jiang, M. et al. Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. J. Anesth. 30, 232–237 (2016).
Wang, J. et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: a randomised double-blind placebo controlled study. Eur. J. Anaesthesiol. 36, 16–24 (2019).
Liu, P. et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J. Invest. Surg. https://doi.org/10.1080/08941939.2019.1710626 (2020).
Zhang, Z. et al. Intraoperative low-dose S-ketamine reduces depressive symptoms in patients with Crohn’s disease undergoing bowel resection: a randomized controlled trial. J. Clin. Med. 12, 1152 (2023).
Aleksandrova, L. R. & Phillips, A. G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 42, 929–942 (2021).
Hess, E. M., Riggs, L. M., Michaelides, M. & Gould, T. D. Mechanisms of ketamine and its metabolites as antidepressants. Biochem. Pharmacol. 197, 114892 (2022).
Johnston, J. N., Henter, I. D. & Zarate, C. A. The antidepressant actions of ketamine and its enantiomers. Pharmacol. Ther. 246, 108431 (2023).
Brown, E. N., Lydic, R. & Schiff, N. D. General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650 (2010).
Heresco-Levy, U. et al. Controlled trial of d-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J. Affect. Disord. 93, 239–243 (2006).
Zarate, C. A. et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 163, 153–155 (2006).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24, 606–615 (2019).
Sanacora, G. et al. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology 42, 844–853 (2017).
Ibrahim, L. et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37, 1526–1533 (2012).
Mathew, S. J., Gueorguieva, R., Brandt, C., Fava, M. & Sanacora, G. A randomized, double-blind, placebo-controlled, sequential parallel comparison design trial of adjunctive riluzole for treatment-resistant major depressive disorder. Neuropsychopharmacology 42, 2567–2574 (2017).
Vesuna, S. et al. Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94 (2020).
Tian, F. et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat. Commun. 14, 1748 (2023).
Chen, X., Shu, S. & Bayliss, D. A. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J. Neurosci. 29, 600–609 (2009).
Anand, A. et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-d-aspartate receptor antagonists. Arch. Gen. Psychiatry 57, 270–276 (2000).
Cichon, J. et al. Ketamine triggers a switch in excitatory neuronal activity across neocortex. Nat. Neurosci. 26, 39–52 (2023).
Williams, N. R. et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am. J. Psychiatry 175, 1205–1215 (2018).
Hustveit, O., Maurset, A. & Oye, I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol. Toxicol. 77, 355–359 (1995).
Klein, M. E., Chandra, J., Sheriff, S. & Malinow, R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc. Natl Acad. Sci. USA 117, 2656–2662 (2020).
Wulf, H. A., Browne, C. A., Zarate, C. A. & Lucki, I. Mediation of the behavioral effects of ketamine and (2R,6R)-hydroxynorketamine in mice by kappa opioid receptors. Psychopharmacology https://doi.org/10.1007/s00213-022-06118-4 (2022).
Marton, T., Barnes, D. E., Wallace, A. & Woolley, J. D. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol. Psychiatry 85, e75–e76 (2019).
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40 (2006).
Chen, M.-H. et al. Using classification and regression tree modelling to investigate treatment response to a single low-dose ketamine infusion: post hoc pooled analyses of randomized placebo-controlled and open-label trials. J. Affect. Disord. 281, 865–871 (2021).
Pennybaker, S. J., Niciu, M. J., Luckenbaugh, D. A. & Zarate, C. A. Symptomatology and predictors of antidepressant efficacy in extended responders to a single ketamine infusion. J. Affect. Disord. 208, 560–566 (2017).
Jesus-Nunes, A. P. et al. Clinical predictors of depressive symptom remission and response after racemic ketamine and esketamine infusion in treatment-resistant depression. Hum. Psychopharmacol. Clin. Exp. 37, e2836 (2022).
Ustun, Y. B. et al. Comparison of ketamine, dexmedetomidine and lidocaine in multimodal analgesia management following sleeve gastrectomy surgery: a randomized double-blind trial. J. Perianesth. Nurs. 37, 820–826 (2022).
Seman, M. T. et al. Low-dose ketamine infusion for perioperative pain management in patients undergoing laparoscopic gastric bypass: a prospective randomized controlled trial. Anesthesiol. Res. Pract. 2021, e5520517 (2021).
Moro, E. T. et al. Ketamine does not enhance the quality of recovery following laparoscopic cholecystectomy: a randomized controlled trial. Acta Anaesthesiol. Scand. 61, 740–748 (2017).
Ragazzoni, L. et al. Intra-operative low-dose ketamine does not reduce the cost of post-operative pain management after surgery: a randomized controlled trial in a low-income country. Afr. Health Sci. 19, 3127–3135 (2019).
Mortero, R. F. et al. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth. Analg. 92, 1465 (2001).
Aubrun, F. et al. Effect of a low-dose ketamine regimen on pain, mood, cognitive function and memory after major gynaecological surgery: a randomized, double-blind, placebo-controlled trial. Eur. J. Anaesthesiol. 25, 97–105 (2008).
Lee, C. et al. The effects of a combination of intravenous dexamethasone and ketamine on postoperative mood in patients undergoing laparoscopically assisted-gynecologic surgery. Psychopharmacology 235, 2417–2422 (2018).
Irle, E., Peper, M., Wowra, B. & Kunze, S. Mood changes after surgery for tumors of the cerebral cortex. Arch. Neurol. 51, 164–174 (1994).
Jenkins, L. M., Drummond, K. J. & Andrewes, D. G. Emotional and personality changes following brain tumour resection. J. Clin. Neurosci. 29, 128–132 (2016).
McGirr, A. et al. Adjunctive ketamine in electroconvulsive therapy: updated systematic review and meta-analysis. Br. J. Psychiatry 210, 403–407 (2017).
Anderson, I. M. et al. Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine–ECT): a multicentre, double-blind, randomised, parallel-group, superiority trial. Lancet Psychiatry 4, 365–377 (2017).
Vlisides, P. E. et al. Neurophysiologic correlates of ketamine sedation and anesthesia: a high-density electroencephalography study in healthy volunteers. Anesthesiology 127, 58–69 (2017).
Rutherford, B. R., Wall, M. M., Glass, A. & Stewart, J. W. The role of patient expectancy in placebo and nocebo effects in antidepressant trials. J. Clin. Psychiatry 75, 1040–1046 (2014).
Kroenke, K. et al. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 114, 163–173 (2009).
Fekadu, A., Donocik, J. G. & Cleare, A. J. Standardisation framework for the Maudsley staging method for treatment resistance in depression. BMC Psychiatry 18, 100 (2018).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33 (1998). quiz 34-57.
Montgomery, S. A. & Åsberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389 (1979).
Zigmond, A. S. & Snaith, R. P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Pinto-Meza, A., Serrano-Blanco, A., Peñarrubia, M. T., Blanco, E. & Haro, J. M. Assessing depression in primary care with the PHQ-9: can it be carried out over the telephone? J. Gen. Inter. Med. 20, 738–742 (2005).
Hedman, E. et al. Telephone versus internet administration of self-report measures of social anxiety, depressive symptoms, and insomnia: psychometric evaluation of a method to reduce the impact of missing data. J. Med. Internet Res. 15, e229 (2013).
Hermens, M. L. et al. Administering the MADRS by telephone or face-to-face: a validity study. Ann. Gen. Psychiatry 5, 3 (2006).
Kobak, K. A., Williams, J. B. W., Jeglic, E., Salvucci, D. & Sharp, I. R. Face-to-face versus remote administration of the Montgomery–Asberg Depression Rating Scale using videoconference and telephone. Depress. Anxiety 25, 913–919 (2008).
Posner, K. et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277 (2011).
Kishimoto, T. et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol. Med. 46, 1459–1472 (2016).
Nierenberg, A. A. & DeCecco, L. M. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J. Clin. Psychiatry 62, 5–9 (2001).
Machado, M., Iskedjian, M., Ruiz, I. & Einarson, T. R. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Curr. Med. Res. Opin. 22, 1825–1837 (2006).
Keller, S. et al. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain 20, 309 (2004).
Mendoza, T. R. et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin. J. Pain 20, 357 (2004).
Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors (US Department of Health and Human Services; 2021); https://www.hhs.gov/guidance/document/opioid-oral-morphine-milligram-equivalent-mme-conversion-factors-0
Hengartner, M. P. & Plöderl, M. Estimates of the minimal important difference to evaluate the clinical significance of antidepressants in the acute treatment of moderate-to-severe depression. BMJ Evid. Based Med. 27, 69–73 (2022).
Acknowledgements
This work was supported by a grant awarded to B.D.H. by the Society for Neuroscience in Anesthesiology and Critical Care. T.R.L. received salary support through a T32 grant from the NIH National Institute on Drug Abuse (3T32DA035165-02S1). The funding bodies supporting this study had no influence on the conduct of the trial, analysis of the data, or reporting of the results. We acknowledge K. Pfaff (medical student, Ohio University Heritage College of Osteopathic Medicine, Athens, OH, USA) and R. Thordstein (Lund University, Lund, Sweden) for assistance with contacting patients and V. Ramachandran (Stanford University School of Medicine, Stanford, CA, USA) for implementing the PHQ-2 survey into the Anesthesia Preoperative Evaluation Clinic electronic workflow at Stanford. Statistical support was provided by Data Studio (Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA, USA), which is supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under award number UL1TR003142. Screening and outcomes data were entered into Stanford REDCap (version 13.4.10), a secure online data-capture platform (http://redcap.stanford.edu) developed and operated by the Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by (1) the Stanford School of Medicine Research Office and (2) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085.
Author information
Authors and Affiliations
Contributions
T.R.L. and B.D.H. designed the trial. T.R.L. analyzed the data and wrote the first draft of the paper. A.E.S., J.R.F., R.L.O., C.A.N. and L.J.C. performed the trial and collected the data. L.M.H. and A.F.S. provided content expertise and advice on trial design. The overall trial was overseen by B.D.H. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Corresponding author
Ethics declarations
Competing interests
B.D.H. is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures. A.F.S. has served as a consultant to Alto Neuroscience, ANeurotech, Compass, Magnus, NeuraWell, Parexal, Sage and Signant. He holds equity in Alto Neuroscience, Corcept, Delpor, Madrigal, Magnus, Seattle Genetics, Titan and Xhale. These interests had no role in the present trial. The other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Gerard Sanacora and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
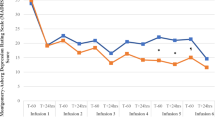
Extended Data Fig. 1 Depression ratings reanalyzed according to patient guess.
On day 14, the final day of patient assessments, patients were asked the following questions: “What treatment do you think you received?” MADRS scores were reanalyzed according to their guess, irrespective of their true group allocation. Mean and standard deviation (SD) MADRS scores are shown using the alternate grouping: “Ketamine”, n = 17; “Placebo”, n = 10; “I don’t know”, n = 11.
Supplementary information
Supplementary Information
Clinical trial protocols, statistical analysis plans and summary of protocol amendments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lii, T.R., Smith, A.E., Flohr, J.R. et al. Randomized trial of ketamine masked by surgical anesthesia in patients with depression. Nat. Mental Health 1, 876–886 (2023). https://doi.org/10.1038/s44220-023-00140-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00140-x
This article is cited by
-
Sub-anesthetic dose of esketamine decreases postoperative opioid self-administration after spine surgery: a retrospective cohort analysis
Scientific Reports (2024)
-
Pharmakotherapie, Psychotherapie oder „Superplacebos“?
Die Psychotherapie (2024)
-
Placebo’s role in the rapid antidepressant effect
Nature Mental Health (2023)