Abstract
Ketamine, a commonly used general anesthetic, can produce rapid and sustained antidepressant effect. However, the efficacy and safety of the perioperative application of ketamine on postoperative depression remains uncertain. We performed a meta-analysis to determine the effect of perioperative intravenous administration of ketamine on postoperative depression. Randomized controlled trials comparing ketamine with placebo in patients were included. Primary outcome was postoperative depression scores. Secondary outcomes included postoperative visual analog scale (VAS) scores for pain and adverse effects associated with ketamine. Fifteen studies with 1697 patients receiving ketamine and 1462 controls were enrolled. Compared with the controls, the ketamine group showed a reduction in postoperative depression scores, by a standardized mean difference (SMD) of −0.97, 95% confidence interval [CI, −1.27, −0.66], P < 0.001, I2 = 72% on postoperative day (POD) 1; SMD−0.65, 95% CI [−1.12, −0.17], P < 0.001, I2 = 94% on POD 3; SMD−0.30, 95% CI [−0.45, −0.14], P < 0.001, I2 = 0% on POD 7; and SMD−0.25, 95% CI [−0.38, −0.11], P < 0.001, I2 = 59% over the long term. Ketamine reduced VAS pain scores on POD 1 (SMD−0.93, 95% CI [−1.58, −0.29], P = 0.005, I2 = 97%), but no significant difference was found between the two groups on PODs 3 and 7 or over the long term. However, ketamine administration distinctly increased the risk of adverse effects, including nausea and vomiting (risk ratio [RR] 1.40, 95% CI [1.12, 1.75], P = 0.003, I2 = 30%), headache (RR 2.47, 95% CI [1.41, 4.32], P = 0.002, I2 = 19%), hallucination (RR 15.35, 95% CI [6.24, 37.34], P < 0.001, I2 = 89%), and dizziness (RR 3.48, 95% CI [2.68, 4.50], P < 0.001, I2 = 89%) compared with the controls. In conclusion, perioperative application of ketamine reduces postoperative depression and pain scores with increased risk of adverse effects.
Similar content being viewed by others
Introduction
Ketamine has been a commonly used general anesthetic in clinical practice for nearly 60 years [1]. It is mainly used for the induction and maintenance of anesthesia, sedation, and analgesia [2,3,4,5]. Outside of these properties, ketamine has also been found to be effective in managing depression, which has always been an important research topic in the past decade. In 2000, a study from Yale University first reported that a single intravenous (IV) injection of ketamine (0.5 mg/kg) exerted rapid-onset and sustained antidepressant effect in patients with depression [6], and several subsequent clinical studies further confirmed ketamine’s robust antidepressant effects in patients with treatment-resistant depression [7,8,9,10,11,12]. Additionally, accumulating evidence has indicated that ketamine rapidly decreases suicide ideation in patients with severe depression [13], which provides a foundation for perioperative administration of ketamine to improve postoperative depression.
Surgical treatment is a great challenge to patients and can induce psychological stress reactions, including anxiety and depression, during the perioperative period that affect postoperative recovery quality and may even increase postoperative complications [14, 15]. Approximately 10–30% of patients experience a depressed mood during the perioperative period [16], especially in patients undergoing cardiac surgery [17]. Patients with underlying depression preoperatively could have worsened the severity of depression after surgery [18, 19]. Additionally, postoperative depression has been described as a significant contributor to postoperative pain, decreased cognitive function, prolonged hospitalization, and morbidity [20,21,22]. Therefore, it is very valuable to investigate how to improve and prevent postoperative depression in patients undergoing surgery.
However, with related studies growing recently, the effect of ketamine on perioperative depression among surgical patients is still controversial. Several clinical trials have demonstrated that the perioperative application of ketamine was associated with improved postoperative depression scores, while some studies showed no significant antidepressant effects [23, 24]. Therefore, we performed a meta-analysis of the antidepression effects and related adverse effects of ketamine during the perioperative period to provide a clinical reference.
Methods
The meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [25]. This study was conducted in accordance with an established protocol and prospectively registered in the international prospective register of systematic reviews (PROSPERO) [26], and the registration information is available at https://www.crd.york.ac.uk/PROSPERO/ (registration number: CRD42020185268).
Literature search and screening
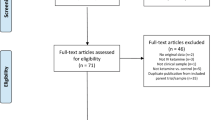
We performed a systematic literature search using the key words “(ketamine OR N-methyl-D-aspartic acid OR NMDA OR glutamate) AND (depression OR depressive OR depressed OR mood) AND (perioperative OR anesthesia OR surgery OR perioperative)” with the limitations “English written”, “clinical trial”, and “randomized controlled trial” in the Cochrane Central Register of Controlled Trials (CENTRAL, including PubMed, Embase, CINAHL, Clinical trials, and the WHO’s International Clinical Trials Registry Platform), Medline, and Web of Science. The search included dates through May 22, 2022, and was conducted by two independent investigators (the initial two authors: J.G. and D.Q.). In the initial screening stage, both investigators screened the titles and abstracts of all articles. Inconsistent selections and disagreements were discussed to reach a consensus. In the subsequent stage of screening for eligibility, the inclusion criteria were as follows: (1) randomized studies that explored the perioperative application of ketamine (experimental group) in comparison with a control group (saline or other drugs) for postoperative depressed mood; and (2) articles on human clinical trials. Articles were excluded if they were (1) reviews, case reports, or nonrandomized studies or (2) included no experimental or control group or the relevant data on the interesting outcomes could not be extracted. The selection protocol is depicted in Fig. 1.
Data extraction
We extracted data from the included studies using Microsoft Excel and then transcribed the data to Review Manager (Version 5.4) for statistical analysis. The study characteristics analyzed included author name, study design, number of patients, surgical type, anesthesia type, time and dose of ketamine intervention, postoperative depression score, pain intensity score, postoperative adverse effects, and follow-up period. We extracted all rating scale scores regarding changes in depressed mood from the included studies as the primary outcome, including the Patient Health Questionnaire (PHQ), Montgomery-Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), Hospital Anxiety and Depression Scale (HADS), Profile of Mood States (POMS), and Edinburgh Postnatal Depression Scale (EPDS) scores. The secondary outcomes were the postoperative visual analog scale pain scores and adverse effects (nausea and vomiting, headache, hallucination, and dizziness). When data were not available in the articles, we attempted to contact the authors to request the original data or use Get Data software to obtain the original data from the figure. Furthermore, if multiple rating scales were used in one study, we gave preference to the MADRS. Four treatment time points were chosen (postoperative days 1, 3, and 7 and over the long term) to assess the effects of ketamine. If multiple time points were tested in one study at 7 days postoperatively, a time point close to 1 month was preferentially selected. Additionally, the study quality and risk of bias were independently assessed by two trained reviewers according to the Cochrane Collaboration’s risk of bias tools [27] (Review Manager 5.4). The risk of bias in each trial was assessed into “low”, “high”, and “some concerns” based on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases. Any discrepancies were resolved via discussion to reach a consensus.
Subgroup analysis
To examine whether the observed effects of ketamine on postoperative depression varied in terms of the moderator variables, the following subgroup analyses were conducted based on the presence of depression preoperatively (with vs. without), type of anesthesia (spinal anesthesia vs. general anesthesia), method of ketamine administration (single-dose vs. continuous infusion administration), dose of ketamine (low vs. high dose) and midazolam premedication (with vs. without). We were unable to perform a subgroup analysis on the effect of (S)-ketamine (or esketamine) and ketamine because there were only one or two studies in each subgroup. To further investigate the safety of intraoperative ketamine for antidepressant use, we performed a subgroup analysis of ketamine-related adverse effects with regard to the method of ketamine administration (single vs. continuous infusion), dose of ketamine (low dose vs. high dose), type of anesthesia (spinal anesthesia vs. general anesthesia) and midazolam premedication (with vs. without).
Statistical analysis
In the present study, the meta-analytic procedures consisted of the following three parts: (a) differences in a postoperative depressive mood, (b) differences in VAS scores, and (c) differences in treatment-related adverse effects. For the continuous variables, the standardized mean difference (SMD) with 95% confidence intervals (95% CIs) was calculated because different scales were employed between studies to measure the same outcome. Dichotomous variables were pooled as the risk ratio with 95% CI. If the trial did not report a particular continuous outcome in the form of a mean and standard deviation, we converted values according to a mature conversion method [28]. We assessed heterogeneity using the I2 value, which estimates the amount of total variation that is attributable to heterogeneity. If the values were <50%, a fixed effects model was chosen. If heterogeneity was >50%, we selected a random effects model and explored sources of heterogeneity using further a subgroup analysis. A two-tailed p value <0.05 was considered to indicate statistical significance.
Results
Study selection and characteristics
The initial internet search yielded 900 potentially relevant studies. After excluding duplicates, 725 studies remained. Among these studies, 692 studies were excluded after reviewing the titles and abstracts. Among the remaining 33 studies, 18 studies were excluded after the two reviewers independently examined the full texts for the following reasons: application of epidural ketamine, no relevant data regarding the postoperative depression outcome, and ongoing studies. Finally, 15 studies that compared ketamine with placebo fulfilled all inclusion criteria and were used to perform the meta-analysis (Fig. 1). Altogether, 15 studies included 3159 adult patients undergoing surgical treatment [23, 24, 29,30,31,32,33,34,35,36,37,38,39,40,41]. Of the 15 eligible trials, there were nine studies from China, one from the USA, one international multicenter study (four countries and ten centers), and one each from Korea, Egypt, Iran, and Japan. Fourteen studies used saline as a control, and one study used dexamethasone as an active control. Four studies included patients with preoperative depression, eight studies excluded patients with preoperative depression, and three studies included patients with no restrictions. Anesthesia types included spinal anesthesia in five studies and general anesthesia in 10 studies. Additionally, four studies received a single dose of ketamine, and nine studies received continued infusion of ketamine. One study used ketamine in patient-controlled intravenous analgesia and one study used two combined methods. Eight studies received high-dose ketamine (≥0.5 mg/kg), and seven studies received low-dose ketamine (<0.5 mg/kg). The main characteristics of the included randomized controlled trials (RCTs) are described in Table 1.
Quality assessment
Of the included trials, four studies did not provide sufficient information regarding random sequence generation, and four studies did not provide adequate details on allocation concealment. One study did not blind the anesthesiologists, and one study did not provide information on the blinding of participants and personnel. We assessed the study quality and risk of bias in accordance with specific conditions. The risk of bias assessment for individual studies and its summary are presented in Fig. 2A, and B, respectively.
Postoperative depression scores
The first part of our main meta-analysis investigated the different effects of ketamine and saline/dexamethasone on depression scores from surgical patients on PODs 1, 3, and 7 over the long term. Altogether, 1697 patients receiving ketamine/esketamine and 1462 patients receiving saline/dexamethasone as controls were extracted from the 15 recruited studies. The antidepressant effect was better in the ketamine group than in the control group. Compared with the placebo group, the ketamine group showed positive effects on POD 1 (SMD − 0.97, 95% CI [−1.27, −0.66], P < 0.001, I2 = 72%), POD 3 (SMD − 0.65, 95% CI [−1.12, −0.17], P < 0.001, I2 = 94%), POD 7 (SMD − 0.30, 95% CI [−0.45, −0.14], P < 0.001, I2 = 0%), and over the long term (SMD−0.25, 95% CI [−0.38, −0.11], P < 0.001, I2 = 59%). Forest plots of this analysis are presented in Fig. 3.
Postoperative pain scores
In the second part of the meta-analysis, we compared the postoperative pain intensity assessed using VAS pain scores between patients receiving ketamine and those receiving control drugs. Our analysis indicated that ketamine was more effective than the placebo in reducing postoperative pain intensity on POD 1 (SMD − 0.93, 95% CI [−1.58, −0.29], P = 0.005, I2 = 97%), whereas no significant difference was observed in the ketamine group on POD 3 (SMD − 0.30, 95% CI [−0.74, −0.14], P = 0.19, I2 = 84%) and POD 7 (SMD − 0.93, 95% CI [−2.37, −0.52], P = 0.21, I2 = 97%). The forest plots of this analysis are presented in Fig. 4.
Adverse effects
Next, we investigated the effects of ketamine on adverse reactions, including nausea and vomiting, headache, hallucinations, and dizziness. The ketamine group had a higher risk of nausea and vomiting (RR 1.40, 95% CI [1.12, 1.75], P = 0.003, I2 = 30%), headache (RR 2.47, 95% CI [1.41, 4.32], P = 0.002, I2 = 19%), hallucination (RR 15.35, 95% CI [6.24, 37.34], P < 0.001, I2 = 89%), and dizziness (RR 3.48, 95% CI [2.68, 4.50], P < 0.001, I2 = 89%) than the control group (Fig. 5).
Subgroup analysis
Postoperative depression scores
Subgroup analysis for postoperative depression scores between patients with and without preoperative depression showed that ketamine had beneficial effects on POD 1 on both patients with (SMD − 0.97, 95% CI [−1.56, −0.38], P = 0.001, I2 = 75%) and without preoperative depression (SMD − 1.01, 95% CI [−1.51, −0.50], P < 0.001, I2 = 81%) compared with placebo (Supplementary Fig. 1A). As most studies on postpartum depression focused on the long-term outcomes after operation, we performed a subgroup analysis of anesthesia type over the long term after operation. According to a subgroup analysis of the anesthesia type, ketamine reduced postoperative depression scores over the long term in patients with spinal anesthesia (SMD − 0.23, 95% CI [−0.41, 0.05], P = 0.01, I2 = 69%) and patients with general anesthesia (SMD − 0.29, 95% CI [−0.52, −0.07], P = 0.01, I2 = 41%) (Supplementary Fig. 1B). Additionally, both single-dose administration and continued infusion of ketamine obviously relieved the postoperative depression scores on POD 1 (SMD − 0.89, 95% CI [−1.27, −0.50], P < 0.001, I2 = 70%; SMD − 1.08, 95% CI [−1.65, −0.51], P < 0.001, I2 = 80%) (Supplementary Fig. 2A). Regarding the intervention dose, ketamine demonstrated remarkable beneficial effects on postoperative depression scores in the low-dose (<0.5 mg/kg) (SMD − 0.93, 95% CI [−1.71, −0.15], P = 0.02, I2 = 85%; 3 studies) and high-dose (≥0.5 mg/kg) groups (SMD − 1.04, 95% CI [−1.46, −0.62], P < 0.001, I2 = 71%; 4 studies), as compared with placebo (Supplementary Fig. 2B). Subgroup analyses showed that midazolam did not affect the effects of ketamine on postoperative depression scores (Supplementary Fig. 3).
Adverse effects
Ketamine increased the risk of nausea and vomiting (Supplementary Fig. 4A), headache (Supplementary Fig. 4B), and hallucination (Supplementary Fig. 5A) compared with placebo in the single-dose groups. Contrarily, there were no significant differences when ketamine was continuously infused (Supplementary Figs. 4, 5). Additionally, ketamine increased the incidence of headache, hallucination, dizziness in patients without midazolam premedication, whereas no significant difference was found in patients with midazolam premedication (Supplementary Figs. 6B, 7A, B). However, there was no significant difference between ketamine group and control group in the incidence of nausea and vomiting whether midazolam premedication is used or not (Supplementary Fig. 6A). Subgroup analyses of adverse effects with regard to anesthesia type showed that ketamine increased the risk of nausea and vomiting (Supplementary Fig. 8A), hallucination (Supplementary Fig. 9A) and dizziness (Supplementary Fig. 9B) in spinal anesthesia group than general anesthesia group. There was no significant difference in the incidence of headache between ketamine group and control group (Supplementary Fig. 8B). These results suggest that continuous infusion administration of ketamine with midazolam or other general anesthetics could reduce the adverse effects of ketamine.
Discussion
This meta-analysis of 3159 participants, including 1697 participants in the ketamine group and 1462 participants in the placebo group, showed that ketamine has a prophylactic effect on postoperative depression. The results can offer valuable evidence for making appropriate pharmacotherapy decisions in clinical practice to improve perioperative depression.
In this meta-analysis, ketamine improved the postoperative depression scores on PODs 1, 3, and 7 over the long term (up to the 42nd day postoperatively). Previous studies have shown that ketamine can produce rapid onset and sustained (>2 weeks) antidepressant effects in treatment-resistant patients with depression after a single-dose administration [42, 43], corroborating the findings of this review. However, the inconsistent results in the meta-analysis should not be ignored. An international, multicenter, double-blind, randomized study showed that intraoperative injection of ketamine did not prevent depression or decrease depressive symptoms after a major surgery in patients aged >60 years [23]. Additionally, Wang et al. [24] found that ketamine did not make a significant difference in postoperative depression scores, which could be attributed to the fact that patients in the study were healthier with quite low baseline MADRS or BDI scores compared with the patients in other studies. Additionally, the MADRS and BDI are more suitable for patients with serious depression rather than with mild depression. A further study is required to clarify whether ketamine might prevent postoperative depression.
The mechanisms underlying ketamine’s antidepressant effects have not been fully elucidated. Accumulating preclinical data suggest that neurotrophic and growth factors such as brain-derived neurotrophic factor (BDNF) and transforming growth factor β plays a key role in the antidepressant effects of ketamine and its enantiomers [42,43,44,45,46]. In clinical studies, Jiang et al. showed that the improvement in patients with postoperative depression after ketamine administration was associated with elevated serum levels of BDNF [29]. The antidepressant effect of ketamine may be linked to its immunomodulatory and anti-inflammatory effects, as the inflammatory cytokine levels are always increased in surgical patients [33, 47, 48]. Yang et al. reported that serum interleukin-6 could be a predictive biomarker for the antidepressant actions of ketamine in treatment-resistant patients with depression [49]. Furthermore, ketamine is increasingly used as an analgesic for perioperative pain. The link between depression and pain is bidirectional, and both act as risk factors for each other [50, 51]. The analgesic effect of ketamine may be associated with its antidepressant effect, as supported by our meta-analysis that showed ketamine reduced pain intensity on POD 1. Nonetheless, a further study on the underlying effect of ketamine on the link between depression and pain is needed. In addition to NMDA receptor, ketamine is known to interact with opioid receptors [52]. Although a clinical study using small sample size suggested the role of opioid receptor in the antidepressant effects of ketamine [53], the role of opioid receptor system is debatable [54, 55]. Given the analgesic effects of ketamine, it seems that opioid receptors may play a role in the mechanisms of action on postoperative pain. Nonetheless, further clinical study using opioid receptor antagonist is needed to ascertain the role of opioid receptor system in the mechanism of ketamine on postoperative depression. To further identify the population suitable for ketamine use, we conducted a subgroup analysis of the antidepressant effect of ketamine according to whether preoperative depression was present and the different anesthesia types. We found that there were no significant differences in ketamine’s antidepressant effect between patients with and without preoperative depression. Furthermore, ketamine had antidepressant effects on both patients who undergone surgery under spinal anesthesia and general anesthesia. Contrarily, a recent meta-analysis by Wang et al. [56] found that ketamine reduced the postoperative depression scores on POD 3 in patients with preoperative depression, whereas no significant difference was found in nonrestrictive studies. The mixed results may be explained by the latter meta-analysis including only two studies [23, 41].
It is not known whether the antidepressant effect of ketamine is affected by concomitant use of general anesthetics. It is difficult to analyze the effect of propofol, volatile anesthetics, and opioids alone on the antidepressant effect of ketamine because these anesthetics were used as most general anesthesia in this meta-analysis. As an alternative, we performed subgroup analyses with regard to spinal and general anesthesia to examine the total influence of general anesthetics. The results showed that there was no significant difference in antidepressant effect of ketamine between spinal anesthesia and general anesthesia. Furthermore, ketamine-related psychiatric adverse effects were observed when it was administered alone [57], but the concurrent administration of benzodiazepine can reduce these psychiatric adverse effects [58]. In this meta-analysis, the subgroup analysis showed that midazolam premedication could reduce the incidence of ketamine-related headache, hallucination, and dizziness. Collectively, the antidepressant effect of ketamine cannot be affected by general anesthetics such as propofol, volatile anesthetics and midazolam, but ketamine-related adverse effects can be ameliorated by midazolam.
The present meta-analysis revealed a higher risk of nausea and vomiting, headache, visual hallucinations, and dizziness in those receiving ketamine than in the controls. Almost all the previous studies have shown a rapid-acting antidepressant effect of ketamine (0.5 mg/kg over 40 min, IV); however, the optimal dose of ketamine for the treatment of depression remains unknown. A recent double-blind, placebo-controlled study using different doses of ketamine (0.1, 0.2, 0.5, 1.0 mg/kg, over 40 min, IV) demonstrated the antidepressant efficacy of high doses (0.5 and 1.0 mg/kg) of ketamine [59]. However, the high doses of ketamine caused more dissociative symptoms and elevated blood pressure than the low doses [59]. The ketamine dose administered in the included patients in the meta-analysis ranged from 0.1 to 1.0 mg/kg, the most commonly used dose was 0.5 mg/kg. It is generally believed that the adverse effects increase with increasing dose, but there was no significant difference in the adverse reactions between the high (≥0.5 mg/kg) and low-dose (<0.5 mg/kg) groups in the current meta-analysis. The possible reason is the multiple effects of anesthesia and surgery on patients, which still needs further study
Additionally, a subgroup analysis of safety found that continuous infusion of ketamine could effectively reduce the adverse effects related to ketamine compared with single-dose ketamine administration while maintaining its antidepressant effect. Thus, it is necessary to pay more attention to the optimal dose and approach of ketamine for postoperative depression. Notably, the (S)-enantiomer of ketamine, esketamine, has been increasingly used in clinical settings, which showed more potent effects than ketamine [60, 61] and has been shown to be effective in treatment-resistant patients with depression [62, 63]. Contrarily, increasing preclinical data suggest that arketamine, the (R)-enantiomer of ketamine, could produce greater potency and longer-lasting antidepressant-like actions in rodents than esketamine and that the side effects of arketamine are lower than those of esketamine and ketamine [55, 64, 65]. A single intravenous infusion of arketamine (0.5 mg/kg) is reported to produce rapid and sustained antidepressant actions in treatment-resistant patients with depression, and the side effects (i.e., psychotomimetic and dissociative effects) of arketamine (0.5 mg/kg) were much lower than those of esketamine (0.2 and 0.4 mg/kg) [63, 66]. Therefore, future research is needed to compare the efficacy of the perioperative application of esketamine and arketamine on postoperative depression.
The findings of the present meta-analysis should be interpreted with caution due to the following limitations, which are mostly related to the weaknesses of the original trials. First, the total sample size was relatively small, and heterogeneity in most analyses was relatively high, which might be due to the fact that most studies included had various types of anesthesia and different administration methods and dosages, although corresponding subgroup analyses were performed. Second, there was a lack of standardization for patient selection and follow-up periods. The assessed time point of postoperative depression varied among studies. Hence, the precise time course of the effect of ketamine on postoperative depression could not be evaluated. Studies used for subgroup analyses with regard to midazolam premedication were limited but it is consistent with previous study [67] that midazolam can reduce the mental adverse effects of ketamine. Finally, the scales used to assess postoperative depression were different, which also increased the sources of heterogeneity. Therefore, higher quality RCTs with a larger sample size are needed to confirm whether perioperative application of ketamine could improve the symptoms of postoperative depression and reduce postoperative pain intensity.
Conclusion
The current meta-analysis indicated that perioperative application of ketamine is effective for reducing postoperative depression scores and pain intensity. However, ketamine increases the risk of nausea and vomiting, headache, hallucination, and dizziness compared with placebo, especially after a single-dose administration. In future clinical practice, the optimal approach for achieving the best antidepressant effect of ketamine with minimal adverse effects remains a major challenge.
References
Hirota K, Lambert DG. Ketamine; history and role in anesthetic pharmacology. Neuropharmacology 2022;216:109171.
Pribish A, Wood N, Kalava A. A review of nonanesthetic uses of ketamine. Anesthesiol Res Pr. 2020;2020:5798285.
Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55:1059–77.
Dzierba AL, Brodie D, Bacchetta M, Muir J, Wasson L, Colabraro M, et al. Ketamine use in sedation management in patients receiving extracorporeal membrane oxygenation. Intensive Care Med. 2016;42:1822–3.
Michelet D, Brasher C, Horlin AL, Bellon M, Julien-Marsollier F, Vacher T, et al. Ketamine for chronic non-cancer pain: A meta-analysis and trial sequential analysis of randomized controlled trials. Eur J Pain. 2018;22:632–46.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46.
Moaddel R, Luckenbaugh DA, Xie Y, Villaseñor A, Brutsche NE, Machado-Vieira R, et al. D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology. 2015;232:399–409.
Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA Jr. A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord. 2015;17:438–43.
Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;243:516–24.
Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–26.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–8.
Takagi H, Ando T, Umemoto T, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Perioperative depression or anxiety and postoperative mortality in cardiac surgery: a systematic review and meta-analysis. Heart Vessels. 2017;32:1458–68.
Rawson KS, Dixon D, Nowotny P, Ricci WM, Binder EF, Rodebaugh TL, et al. Association of functional polymorphisms from brain-derived neurotrophic factor and serotonin-related genes with depressive symptoms after a medical stressor in older adults. PLoS One. 2015;10:e0120685.
Orri M, Boleslawski E, Regimbeau JM, Barry C, Hassler C, Gregoire E, et al. Influence of depression on recovery after major noncardiac surgery: a prospective cohort study. Ann Surg. 2015;262:882–9.
Foss-Nieradko B, Stepnowska M, Piotrowicz R. Effect of the dynamics of depression symptoms on outcomes after coronary artery bypass grafting. Kardiol Pol. 2012;70:591–7.
Horne D, Kehler S, Kaoukis G, Hiebert B, Garcia E, Duhamel TA, et al. Depression before and after cardiac surgery: do all patients respond the same? J Thorac Cardiovasc Surg. 2013;145:1400–6.
Eshmawey M, Arlt S, Ledschbor-Frahnert C, Guenther U, Popp J. Preoperative depression and plasma cortisol levels as predictors of delirium after cardiac surgery. Dement Geriatr Cogn Disord. 2019;48:207–14.
Patel AK, Biagas KV, Clarke EC, Gerber LM, Mauer E, Silver G, et al. Delirium in children after cardiac bypass surgery. Pediatr Crit Care Med. 2017;18:165–71.
Drudi LM, Ades M, Turkdogan S, Huynh C, Lauck S, Webb JG, et al. Association of depression with mortality in older adults undergoing transcatheter or surgical aortic valve replacement. JAMA Cardiol. 2018;3:191–7.
Elsamadicy AA, Adogwa O, Lydon E, Sergesketter A, Kaakati R, Mehta AI, et al. Depression as an independent predictor of postoperative delirium in spine deformity patients undergoing elective spine surgery. J Neurosurg Spine. 2017;27:209–14.
Mashour GA, Ben Abdallah A, Pryor KO, El-Gabalawy R, Vlisides PE, Jacobsohn E, et al. Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Br J Anaesth. 2018;121:1075–83.
Wang J, Echevarria GC, Doan L, Ekasumara N, Calvino S, Chae F, et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: A randomised double-blind placebo controlled study. Eur J Anaesthesiol. 2019;36:16–24.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–37.
Jiang M, Wang MH, Wang XB, Liu L, Wu JL, Yang XL, et al. Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. J Anesth. 2016;30:232–7.
Lee C, Lee J, Lee G, Lee H, Shicheng Z, Hwang J. The effects of a combination of intravenous dexamethasone and ketamine on postoperative mood in patients undergoing laparoscopically assisted-gynecologic surgery. Psychopharmacology. 2018;235:2417–22.
Mostafa RH, Khamis AM, Saleh AN, Mekki YMH, Kamal MM, Ibrahim IM, et al. Acute effects of ketamine infusion on postoperative mood scores in patients undergoing dilation and curettage: a randomized double-blind controlled study. Anesthesiol Res Pr. 2021;2021:6674658.
Alipoor M, Loripoor M, Kazemi M, Farahbakhsh F, Sarkoohi A. The effect of ketamine on preventing postpartum depression. J Med Life. 2021;14:87–92.
Ren Q, Hua L, Zhou X, Cheng Y, Lu M, Zhang C, et al. Effects of a single sub-anesthetic dose of ketamine on postoperative emotional responses and inflammatory factors in colorectal cancer patients. Front Pharm. 2022;13:818822.
Yao J, Song T, Zhang Y, Guo N, Zhao P. Intraoperative ketamine for reduction in postpartum depressive symptoms after cesarean delivery: A double-blind, randomized clinical trial. Brain Behav. 2020;10:e01715.
Zhou Y, Sun W, Zhang G, Wang A, Lin S, Chan MTV, et al. Ketamine alleviates depressive symptoms in patients undergoing intracranial tumor resection: a randomized controlled trial. Anesth Analg. 2021;133:1588–97.
Ma JH, Wang SY, Yu HY, Li DY, Luo SC, Zheng SS, et al. Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section. Psychiatry Res. 2019;279:252–8.
Han Y, Li P, Miao M, Tao Y, Kang X, Zhang J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. 2022;22:49.
Liu P, Li P, Li Q, Yan H, Shi X, Liu C, et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Invest Surg. 2021;34:883–8.
Kudoh A, Takahira Y, Katagai H, Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg. 2002;95:114–8.
Xu R, Zhan Y, Chen S Effect of intraoperative single administration of sub-anesthesia ketamine on breast cancer patients with depression. Allied Academies, 2017.
Xu Y, Li Y, Huang X, Chen D, She B, Ma D. Single bolus low-dose of ketamine does not prevent postpartum depression: a randomized, double-blind, placebo-controlled, prospective clinical trial. Arch Gynecol Obstet. 2017;295:1167–74.
Hashimoto K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharm. 2020;177:113935.
Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry Clin Neurosci. 2019;73:613–27.
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632.
Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28.
Yao W, Cao Q, Luo S, He L, Yang C, Chen J, et al. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry. 2022;27:1618–29.
Cho JS, Kim NY, Shim JK, Jun JH, Lee S, Kwak YL. The immunomodulatory effect of ketamine in colorectal cancer surgery: a randomized-controlled trial. Can J Anaesth. 2021;68:683–92.
Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065.
Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry. 2015;77:e19–e20.
Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131:438–44.
Wang S, Cardieri B, Mo Lin H, Liu X, Sano M, Deiner SG. Depression and anxiety symptoms are related to pain and frailty but not cognition or delirium in older surgical patients. Brain Behav. 2021;11:e02164.
Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 1999;90:174–82.
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15.
Hashimoto K. Are NMDA and opioid receptors involved in the antidepressant actions of ketamine? Proc Natl Acad Sci USA. 2020;117:11200–1.
Zhang JC, Yao W, Hashimoto K. Arketamine, a new rapid-acting antidepressant: A historical review and future directions. Neuropharmacology 2022;218:109219.
Wang J, Sun Y, Ai P, Cui V, Shi H, An D, et al. The effect of intravenous ketamine on depressive symptoms after surgery: A systematic review. J Clin Anesth. 2022;77:110631.
Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78.
White PF, Way WL, Trevor AJ. Ketamine-its pharmacology and therapeutic uses. Anesthesiology 1982;56:119–36.
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25:1592–603.
Trimmel H, Helbok R, Staudinger T, Jaksch W, Messerer B, Schöchl H, et al. S(+)-ketamine: Current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130:356–66.
Miziara LE, Simoni RF, Esteves LO, Cangiani LH, Grillo-Filho GF, Paula AG. Efficacy of continuous S(+)-ketamine infusion for postoperative pain control: a randomized placebo-controlled trial. Anesthesiol Res Pr. 2016;2016:6918327.
Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–20.
Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–31.
Zhang K, Yao Y, Hashimoto K. Ketamine and its metabolites: Potential as novel treatments for depression. Neuropharmacology 2023;222:109305.
Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. 2022;27:559–73.
Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271:577–82.
Suzuki M, Tsueda K, Lansing PS, Tolan MM, Fuhrman TM, Sheppard RA, et al. Midazolam attenuates ketamine-induced abnormal perception and thought process but not mood changes. Can J Anaesth. 2000;47:866–74.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (No. 82171189) and the Programme of Introducing Talents of Discipline to Universities of Henan (No. CXJD2019008). We thank Yutuan Wu, Jiaying Yang, and Liyun Jiang for their assistance in statistical analysis.
Author information
Authors and Affiliations
Contributions
JG and DQ have access to all the study data and assume responsibility for the data integrity. HG, XW, KH, GZ, and JY contributed to the study’s conception, design, and manuscript writing. JG and DQ performed the statistical analysis. KH, GZ, and JY supervised the study. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Hashimoto is the inventor of filed patent applications on “The use of R-ketamine in the treatment of psychiatric diseases”, “(S)-norketamine and salt thereof as pharmaceutical”, “R-ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder”, “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases”, “R-ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder”, and “TGF-β1 in the treatment of depression” by Chiba University. Dr. K. Hashimoto has also received speakers’ honoraria, consultant fees, or research support from Abbott, Boehringer Ingelheim, Daiichi-Sankyo, Meiji Seika Pharma, Seikagaku Corporation, Sumitomo Pharma, Taisho, Otsuka, Murakami Farm and Perception Neuroscience. The other authors declare that they have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Qiu, D., Gu, Hw. et al. Efficacy and safety of perioperative application of ketamine on postoperative depression: A meta-analysis of randomized controlled studies. Mol Psychiatry 28, 2266–2276 (2023). https://doi.org/10.1038/s41380-023-01945-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01945-z
This article is cited by
-
Randomized trial of ketamine masked by surgical anesthesia in patients with depression
Nature Mental Health (2023)
-
Perioperative Use of Ketamine
Current Pain and Headache Reports (2023)