Abstract
Ovarian hormones have substantial effects on the brain, and early menopause has been associated with increased risk of accelerated brain aging and dementia later in life. However, the impact of ovarian hormone fluctuations on brain structure earlier in life is less understood. Here we show that ovarian hormone fluctuations shape structural brain plasticity during the reproductive years. We use longitudinal ultra-high field neuroimaging across the menstrual cycle to map the morphology of medial temporal lobe subregions in 27 participants. Controlling for water content and blood flow, our findings reveal positive associations between estradiol and parahippocampal cortex volume, progesterone and subiculum and perirhinal area 35 volumes, and an estradiol*progesterone interaction with CA1 volume. This research offers a blueprint for future studies on the shared dynamics of the brain and ovarian function and a fundamental stepping stone towards developing sex-specific strategies to improve brain health and mental health.
Similar content being viewed by others
Main
Ovarian hormones are powerful modulators of neuroplasticity, with animal research offering robust evidence of endocrine regulation of brain morphology on a rapid timescale1. In the timescale of hours to days, rodent and non-human primate studies have demonstrated that estradiol and progesterone elicit modulatory effects on cell proliferation1, dendritic spine and synapse density2,3,4,5, mitochondrial and synaptic health6,7, synaptic sprouting and axon growth8,9,10, and myelination11, suggesting a pivotal role of ovarian hormones in brain structural organization. In humans, the menstrual cycle provides an opportunity to study how endogenous fluctuations in hormones may transiently influence the brain, as estradiol levels increase eightfold and progesterone levels 80-fold over a period of ~25–32 days12. Although a growing number of menstrual cycle studies suggest that ovarian hormone fluctuations do influence brain function and behavior in humans13,14,15, it remains less clear how endocrine factors may shape brain structure following the rhythmic nature of the menstrual cycle, and the implications this would have for human adult neuroplasticity.
In this context, the hippocampus is a key region shown to display a remarkable degree of neuroplasticity during the reproductive years, such as during pregnancy16,17,18,19,20 and during the menstrual cycle21,22,23. The hippocampus is also implicated in emotional regulation and cognition1,24,25, domains that are susceptible to cycle-dependent fluctuations14,15. The hippocampus and extended medial temporal lobe (MTL) are rich in estradiol and progesterone receptors26,27, and previous studies suggest that estradiol-dominant menstrual cycle phases are associated with greater hippocampal volume21,22,23. Findings have been inconsistent, however, as menstrual cycle studies typically only assess two timepoints and do not directly measure ovarian hormone levels, rather using cycle phase as a proxy for hormone states22,23. Recently, a different approach using dense-sampling of hormones and brain across the menstrual cycle has been developed. Compared to sparse-sampling approaches, which typically just measure cycle phases corresponding to peak/trough hormone concentrations, dense-sampling designs assess many menstrual cycle stages to better capture dynamic interactions between the endocrine and nervous systems. For example, in a single-subject pilot study28, we observed that subtle gray matter density changes in the human hippocampus paralleled daily fluctuations in endogenous estradiol levels, while intrinsic functional connectivity changes were associated with endogenous progesterone levels across the menstrual cycle29, a dynamic pattern that would have been overlooked with a sparse-sampling approach. The ‘28andMe’ project30 has also elegantly shown that dense-sampling (n = 1 female participant, sampled 30 days across the full menstrual cycle, 30 days on a hormonal contraceptive regimen) can uncover unique brain–hormone interactions in brain function13,31,32 and structure33 that would otherwise be overlooked, setting the stage for additional studies in larger cohorts. Thus, the hippocampus and surrounding MTL are promising targets for cycle-related hormonal modulation of structural brain plasticity, but study designs require densely sampled hormone and neuroimaging data over the timescale of the entire menstrual cycle to best capture intra- and interindividual variability in both cycle variation and brain structure.
Moreover, although most human magnetic resonance imaging (MRI) studies treat the hippocampus as a homogeneous structure, recent advances in neuroimaging allow for more precise delineation of neuroanatomical subregions of the hippocampus and MTL in vivo in humans34,35,36. This specificity is important given the unique cytoarchitecture, chemoarchitecture and circuity of MTL subregions36,37,38 that differentially contribute to aging and disease39,40. For example, previous studies have shown that visual memory is more dependent on the perirhinal cortex, that spatial memory is more dependent on the parahippocampal cortex, that the subiculum plays an important role in mediating hippocampal–cortical communication, and that the hippocampus is crucial for both semantic memory and episodic memory but that lesion location within the hippocampus uniquely impairs these memory types (reviewed in refs. 41,42). Subregion-specific architecture and circuitry, alongside potential differences in hormone receptor densities43, suggest that hormone-modulated volumetric changes may manifest differently across the MTL complex, such as has been recently shown in women with early oophorectomy44. Supporting evidence for the influence of ovarian hormone fluctuations on subregions mainly stems from animal work, with particular emphasis on the cornu ammonis 1 (CA1), a subregion critical for memory integration45 and in which neuronal loss has been associated with Alzheimer’s disease in an all-male human study46. Estradiol administration enhances synaptogenesis and spine density in CA1 neurons of ovariectomized adult rodents2,4,47 and ovariectomized young and aged non-human primates48, whereas progesterone inhibits this effect. A study in ovariohysterectomized young-to-middle-aged primates found that estradiol treatment increases pre- and post-synaptic proteins in CA1, whereas combined estradiol and progesterone decreases these synaptic proteins49. As women have a greater risk of developing Alzheimer’s disease relative to men50, an additional region of interest is perirhinal area 35, a subregion in which atrophy has been associated with cognitive decline as well as early stages of dementia in older adult mixed-sex studies that either controlled51,52 or did not control for sex53,54,55. Area 35, corresponding to the transentorhinal region and medial perirhinal cortex34,55, has thus far received little attention regarding endogenous ovarian hormone fluctuations. This constitutes a critical knowledge gap given the relevance of this subregion to dementia progression and that changes in hormone status have been proposed to underlie sex differences in Alzheimer’s disease pathology56,57,58. Hence, investigating the influence of subtle hormone fluctuations on CA1 and area 35 volume is probably highly relevant for a mechanistic understanding of female-specific cognitive decline risk.
Initial evidence for hormone-modulated changes across human MTL subregions has been observed in a recent single-subject study using 3-T MRI, in which the authors observed associations between daily ovarian hormone levels and MTL complex volumes33. No study has yet applied a high-density sampling protocol to test whether consistent patterns of hormone–volume associations at the subregion level can be identified in the human hippocampus and surrounding MTL in multiple participants across the menstrual cycle. To shed further light on hormone-associated hippocampal and MTL changes in the female human brain during the reproductive years, we provide a densely sampled and detailed ultra-high field neuroimaging dataset in 27 healthy participants who underwent 7-T MRI scanning during six menstrual cycle phases: menstrual, pre-ovulatory, ovulation, post-ovulatory, mid-luteal and premenstrual. We utilized the Automated Segmentation of Hippocampal Subfields software (ASHS35), which allows for a sensitive approach to individual differences in MTL subregion morphology59. Notably, the chosen Magdeburg Young Adult 7T Atlas34 leverages information on anatomical variability, resulting in a more accurate delineation of the boundary between the CA1 and subiculum, CA3 and dentate gyrus, as well as the parahippocampal cortex, and further segmentation of the perirhinal region into areas 35 and 36. These advantages, alongside 7-T MRI images with small slice thickness and high in-plane resolution, allow for better delineation and segmentation of the MTL subregions. We also developed a systematic protocol for rigorous cycle phase characterization to overcome the inaccuracy of menstrual cycle monitoring, a limitation of previous work in this field60,61,62. Based on our pilot study28, we hypothesized that cycle-related increases in estradiol levels would be associated with increases in whole hippocampus volume. Within the subregions, based on the above-mentioned animal literature, we hypothesized that estradiol levels would be positively associated with perirhinal area 35 volume, and that there would be an interaction between estradiol and progesterone levels in CA1 volume. Other subregion volumes (CA2, CA3, subiculum, dentate gyrus, area 36, entorhinal and parahippocampal cortices) were assessed in an exploratory fashion. Given the essential role of the hippocampus and MTL in adult neuroplasticity, these findings may contribute to a better understanding of how endocrine factors shape healthy adult brain dynamics during the reproductive years as well as inform more individualized strategies for neuroimaging the female brain.
Results
Monitoring
All baseline demographics are reported in Table 1. Participants were of reproductive age, with healthy body mass index (BMI) and regular menstrual cycle length. Endogenous ovarian hormone values, subregion volumes and whole hippocampus volume (sum of CA1, CA2, CA3, subiculum, dentate gyrus and remaining tail) were within the expected ranges (Fig. 1 and Table 2). For further analyses, hormone values were log-transformed and bilateral subregion volumes were adjusted for total brain volume, and statistical significance was accepted at a Benjamini–Hochberg false detection rate (FDR) corrected threshold of q < 0.05 (see Methods for additional monitoring and statistical details).
Control analyses
Previous work in the field has been critiqued for not taking into account potential hormone-related water shifts or cerebral blood flow (CBF) changes in the brain, which could be misconstrued as hormone-related brain-volume changes. Although the Magdeburg Young Adult 7T Atlas does exclude the alveus, fimbria, cerebrospinal fluid (CSF) and blood vessels, we additionally assessed CSF and CBF changes in the hippocampus and did not observe statistically significant associations with estradiol (CSF: β = 4.28, 95% CI = −14 to 6, random effects s.d. = 22.62, P = 0.402; CBF: β = 1.23, 95% CI = −3 to 1, random effects s.d. = 4.19, P = 0.195) or progesterone (CSF: β = 3.67, 95% CI = −1 to 8, random effects s.d. = 22.43, P = 0.134; CBF: β = 0.06, 95% CI = −1 to 1, random effects s.d. = 4.22, P = 0.899), giving further confidence that the following results were not erroneously driven by these factors.
The bilateral precentral gyrus was identified as a control region outside the MTL, as this brain region is not reported to be strongly influenced by fluctuations in ovarian hormones. We did not observe statistically significant associations between precentral gyrus volume with estradiol nor with progesterone (estradiol: β = 3,477.33, 95% CI = −747 to 702, random effects s.d. = 9,415.84, P = 0.106; progesterone: β = −161.23, 95% CI = −2,232 to 1,910, random effects s.d. = 9,564.48, P = 0.878).
Ovarian hormones and MTL volumes across the menstrual cycle
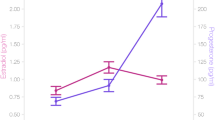
As hypothesized, linear mixed-effects modeling showed positive associations between estradiol levels and whole hippocampus volume (β = 108.26, 95% CI = 27 to 190, random effects s.d. = 174.47, P = 0.009). In MTL subregions, addition of the estradiol*progesterone interaction to the model significantly improved model fit only for CA1 (Fig. 2a; χ2(1) = 7.691, P = 0.006). Estradiol was positively associated with CA1 volume, progesterone was negatively associated with CA1 volume, and we observed a significant interaction of estradiol and progesterone with CA1 volume (estradiol: β = 42.87, 95% CI = 21 to 65, P < 0.001; progesterone: β = −150.02, 95% CI = −249 to −51, P = 0.003; interaction: β = 53.06, 95% CI = 16 to 90, random effects s.d. = 44.03, P = 0.005), such that at higher progesterone levels, the positive effect of estradiol on CA1 volume was attenuated. Progesterone was positively associated with subiculum volume (Fig. 2b) and with area 35 volume (Fig. 2c) (subiculum: β = 13.12, 95% CI = 4 to 22, random effects s.d. = 43.29, P = 0.006; area 35: β = 11.98, 95% CI = 2 to 21, random effects s.d. = 44.01, P = 0.014). Finally, estradiol was positively associated with parahippocampal cortex volume (Fig. 2d; β = 24.33, 95% CI = 10 to 39, random effects s.d. = 32.48, P = 0.001). All four subregions showed significant changes in volume over the six cycle phase timepoints (Fig. 2, column 2), but such cycle phase effects did not survive correction for multiple comparisons in the whole hippocampus or subiculum. We did not observe any significant relationship between hormones and CA2, CA3, dentate gyrus, entorhinal cortex or area 36 volumes (0.196 ≤ P ≤ 0.884) (Fig. 1).
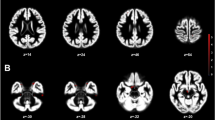
a–d, Column 1: example T2-weighted image, T1-weighted image and MTL segmentation for CA1 (a), subiculum (b), perirhinal area 35 (c) and parahippocampal cortex (d). Column 2: after segmentation, unique associations between ovarian hormones and MTL regions across the menstrual cycle (Mens, menstrual; Pre-O, pre-ovulatory; Ovul, ovulation; Post-O, post-ovulatory; Mid-L, mid-luteal; Pre-M, premenstrual). Linear mixed-effects model statistics are shown (with uncertainty intervals (equal-tailed) and P values (two-tailed) computed using a Wald t-distribution approximation, and corrected for multiple comparisons using the Benjamini–Hochberg procedure at an FDR-corrected threshold of q < 0.05). The center of the error band represents the mean, with the shaded area representing +95% CI based on the bootstrap distribution for hormone levels or subregion volumes. Asterisks refer to statistically significant changes (corrected for multiple comparisons) in volume over timepoints. Details are as follows. a, CA1: Mens versus Pre-O, P = 0.001; Mens versus Post-O, P = 0.006; Pre-O versus Mid-L, P = 0.022; Pre-O versus Pre-M, P = 0.025. c, Area 35: Mens versus Mid-L, P = 0.004; Pre-O versus Mid-L, P = 0.007; Mens versus Post-O, P = 0.025. d, Parahippocampal cortex: Mens versus Mid-L, P = 0.0001; Mid-L versus Pre-M, P = 0.005; Mens versus Pre-O, P = 0.007; Mens versus Post-O, P = 0.02; Mens versus Pre-O, P = 0.042. *P < 0.05, **P < 0.005. N = 27.
Discussion
In this Article we have combined dense-sampling of hormones and brain with individually derived high-resolution MTL segmentation analysis to demonstrate how estradiol and progesterone fluctuations affect key memory regions, such as the MTL and hippocampal subregion volumes. Parahippocampal cortex, area 35, subiculum and CA1 volumes showed significant changes in association with hormone fluctuations across the menstrual cycle. More specifically, estradiol levels were positively associated with parahippocampal cortex volume, and progesterone levels were positively associated with subiculum and area 35 volume. We also observed an estradiol*progesterone level interaction with CA1 volume. The observed volumetric changes and their unique associations with ovarian hormones, especially progesterone, were concealed when analyzing the hippocampus as a whole, a potential limitation for neuroimaging studies that traditionally assess the hippocampus as a single homogeneous structure. We provided further confidence that the observed effects do not erroneously stem from extra-neuronal factors, such as hormone-induced changes in regional CBF or CSF expansion, as we did not find associations between these factors and ovarian hormone levels. Finally, we also did not observe any associations between ovarian hormone levels and brain volume in our control region, the precentral gyrus. Taken together, our results suggest that ovarian hormones rapidly alter structural brain plasticity during the reproductive years and that hippocampal and MTL subregions may be differentially sensitive to hormones.
We were especially interested in CA1 and area 35 given previously observed selective patterns of neuronal vulnerability to memory impairment in CA1 in an adult all-male study46 and area 35 in older adult mixed-sex studies51,52,53,54,55, as women are more likely to suffer from cognitive impairment when ovarian hormones rapidly fluctuate, such as during perimenopause57,58,63. The hypothesized interaction in CA1 is in line with previous animal work, showing that estradiol enhances synaptogenesis, spine density and synaptic protein levels in CA1, while subsequent increases in progesterone seem to inhibit this effect2,4,7,47,48,49. Moreover, progesterone administration decreases dendritic spines in ovariectomized adult rodent CA1 neurons, an effect that can be inhibited with a progesterone receptor antagonist2. Although studies in humans are limited and larger brain volume does not necessarily imply better function, we do know that CA1 plays a distinct functional role in memory integration and inference45. Clinical studies in post-menopausal women have also shown that estrogen replacement is associated with maintaining cognitive function in older age, and progesterone may counteract the benefit of estradiol’s cognitive enhancing effect64,65,66. We note, however, that although most hormone replacement therapy studies suggest that estrogen replacement has a beneficial effect on cognition, literature on progesterone is less consistent, with some studies finding positive effects of progesterone67 and other studies finding little, no or negative effects of progesterone on cognitive function64,65,66,68. Thus, our CA1 findings are consistent with previous animal and clinical work suggesting a proliferative effect of estradiol and suppressive effect of progesterone on synaptic plasticity in CA1, a region critical for cognitive processes. Furthermore, as reviewed in the discussion of our next finding, progesterone may have differing effects on brain regions relevant to cognition.
We also observed a positive association between progesterone levels and area 35 volume, which corresponds to the transentorhinal region and medial perirhinal cortex34,55. This region is clinically relevant with regard to aging and disease, with previous work showing that neurodegeneration and atrophy in this region is associated with early stages of dementia and cognitive decline in mixed-sex studies51,52,53,54,55. Given the increasing focus on the interplay between endogenous hormone fluctuations, the MTL and the disproportionate risk for Alzheimer’s disease in women50,58,63, we hypothesized that estradiol fluctuations would be associated with area 35 volumetric changes. The majority of related literature on hippocampal structural plasticity has thus far focused on estradiol relative to progesterone, and the CA1 and dentate gyrus relative to other MTL regions. With the observed progesterone association in this study, additional emphasis on progesterone in future research is warranted given that its levels change ~80-fold over the menstrual cycle12, and that both our current findings and previous work33 show a complex role of progesterone on MTL subregion volumes. We note that, although the single-subject study33 also observed both positive and negative associations between progesterone and subregion volumes, our observed progesterone findings occurred in different areas of the MTL, whereas the other study observed associations with CA2/3, perirhinal, entorhinal and parahippocampal cortex volumes. These differences may be partially driven by differences in the study design and methodology, such as the number of participants, scanner strength, segmentation atlas used and differences in timepoints. Comparing effect sizes in our current study to previous work remains difficult, as the mentioned study33 is the only other study to measure changes in MTL subregion volume across the menstrual cycle, and differences in study design and methodology (especially 3-T versus 7-T) make direct comparison challenging (although we do note that in ref. 33 ~8% volume change in CA1 from menses to pre-ovulatory stages was observed, and we observed a comparable 5%). We therefore encourage replication of the current findings in future studies.
Beyond the hypothesized regions, we also observed positive associations between estradiol and parahippocampal cortex volume, and progesterone and subiculum volume. Although the effect of menstrual cycle timepoint did not survive correction for multiple comparisons in the subiculum, changes in subiculum and parahippocampal cortex volumes have been observed during pregnancy17,18 and after surgical menopause69, both of which are times of more extreme changes in ovarian hormones. The subiculum has also been previously shown to display sex-specific volumetric changes in healthy cognitive aging39 and plays an important role in mediating hippocampal–cortical interactions42, which are critically involved in cognitive function and emotional regulation, processes influenced by menstrual cycle phase14,15. Although we highlight the relevant strengths of the methods used in this Article (for example, an atlas with the distinction of area 35 and a better boundary of subiculum and parahippocampal cortex34), future research is required to replicate the current findings in these subregions, as our study clearly demonstrates complex interactions between ovarian hormones and MTL structure as a first step in understanding hormone-induced modulation of brain plasticity at the subregion level.
Although the nature of our MRI study cannot directly evaluate the physiological processes underlying MTL morphology changes, we can speculate on potential hormone-induced molecular and cellular mechanisms that may contribute to mesoscopic changes in regional brain volume. Animal research lends proof that ovarian hormones serve as critical components of cell survival and plasticity, where possible mechanisms at the microanatomical level could include cell proliferation and microglial activation1,70, dendritic spine and synapse density2,3,4,5, mitochondrial and synaptic health6,7, synaptic sprouting and axon growth8,9,10, and myelination11, which can occur in a matter of hours to days. These hormone-induced actions can occur through activation of classical estrogen (ERα and ERβ) and progesterone receptors (PRs), which are densely found in the MTL complex26,27. For example, previous work has shown that ER antagonists and agonists can respectively decrease and increase cell proliferation in the dentate gyrus of ovariectomized adult rodents71,72. We note that we and others33,49 did not, however, observe volumetric changes in this region across the menstrual cycle, suggesting that the MTL changes we observe may be more driven by synaptic plasticity and remodeling in regions such as the CA1 as opposed to neurogenesis in the dentate gyrus. Indeed, within the hippocampal complex, these three classical receptors appear most prominently in the CA143, where estradiol administration and ER agonists increase synaptic proteins49,73, ER antagonists decrease synaptic proteins74, and progesterone administration and PR antagonists respectively decrease and increase dendritic spines2,4. These modulatory effects were not as prominent in the dentate gyrus and other subregions as in CA1 (refs. 43,49,73,74). Given that previous work has shown that expression of such receptors varies across MTL subregions and across the estrous cycle43, this may provide a mechanism for hormone-related changes in molecular and cellular processes that contribute to MTL subregion-specific volumetric changes observed across the menstrual cycle. However, given differences in scale, these synaptic-level mechanisms cannot fully account for the observed changes in subregion volumes. Our study thus encourages future investigation of the specific biological microstructural mechanisms underlying the observed short-term dynamics of human MTL subregion morphology during times of ovarian hormone fluctuations.
Although the rigorous cycle monitoring protocol and individually derived MTL segmentation analyses serve as strengths of this sufficiently powered longitudinal study, several limitations should be acknowledged. We recruited a healthy population to reflect how endogenous endocrine factors regulate healthy adult brain structural plasticity in the female reproductive years. Most of the reviewed background literature, however, is based on studies using exogenous hormone manipulation and/or populations with a different reproductive status, such as ovariectomized animals or post-menopausal female adults. Reproductive phase (for example, menstrual cycle versus menopause), type of hormone change (for example, endogenous fluctuations versus exogenous manipulation) and timing (for example, years since menopause) can all influence how the brain reacts to changes in ovarian hormone status1,68,75,76. For example, the benefit of hormone therapy on cognitive health and potentially hippocampal plasticity depends on being initiated near the time of cessation of ovarian function76. Therefore, future studies should test whether the observed menstrual-cycle hormone-induced changes in MTL subregions are further exacerbated during times of more profound neuroendocrine change, such as during perimenopause or early oophorectomy, and whether there are behavioral consequences for patient populations. Second, selection bias could emerge if participation is limited by hesitation to participate in multiple neuroimaging sessions and/or rigorous menstrual-cycle monitoring procedures. Given the longitudinal design of the study and the multiple assessments, this cannot be ruled out. The strengths of this within-subject design include higher statistical power and better control of participants’ characteristics or individual differences, such as head motion affecting the individual study outcomes. We also acknowledge the possibility of attrition bias, which is inherent to protocols with multiple follow-up appointments. By careful randomization and controlling for socio-demographic and lifestyle factors, such as education level and perceived stress, we aimed to limit the impact of such bias. Due to the majority of the population in Saxony being of German ethnicity, our sample is 100% European/German. As results from one population may not extrapolate well to another, we need further research to expand neuroimaging studies to enhance representation of multiple populations, and prioritize samples of non-European descent. Finally, we studied an all-female sample. This limits the generalizability of our findings to the male population, but provides relevant information that has been lacking in the neuroscience literature.
Furthermore, recent work does suggest that early changes in neuropsychiatric and neurodegenerative disease are better detected in smaller subregions of the MTL rather than with whole-structure analysis51,52,53,77, and women are at increased risk for such disorders following ovarian hormone fluctuations, such as depressive disorders78,79,80,81, multiple sclerosis82,83,84, dementia57,58 and other autoimmune disorders85, during the premenstrual phase78,79,82,85, postpartum period78,80,83 and following perimenopause57,58,78,81,84. Given the essential role of the MTL in adult synaptic plasticity and the differential contributions of MTL subregions to cognitive functions45,86,87,88,89, this information may be particularly relevant for plasticity-related disorders such as depression and dementia, which women are at greater risk of developing50,90,91. Furthermore, although we aimed to assess endogenous ovarian hormones, future studies should also consider having a control group in which endogenous estradiol and progesterone fluctuations are effectively clamped, such as a hormonal contraception group. Finally, while our analyses focused on the hippocampal and MTL complex, ovarian hormones have widespread effects on many brain areas that work in tandem. Subregions have distinct connectivity profiles37, and future studies should extend the focus to other brain areas to capture a broader network understanding. Given that we saw unique hormone interactions with the CA1 and subiculum, and that hippocampal projections to the medial prefrontal cortex (PFC) originate primarily in the CA1 and subiculum92, we suggest investigation of hormone-modulated changes in structural and functional connectivity between these subregions and the PFC. The PFC also densely expresses hormone receptors93 and displays hormone-induced structural plasticity3,6,7, with the hippocampal–prefrontal pathway implicated in cognitive and emotional processes42,92.
In conclusion, our study incorporated several factors to account for external validity (for example, a longitudinal design that enabled the examination of within-subject changes over time, and the utilization of advanced neuroimaging techniques, such as ultra-high field imaging and individually derived segmentation analysis), contributing to more robust and reliable findings. Most critically, however, this study addresses the considerable gap of missing female data in the neurosciences, thus providing a substantial contribution to improving the generalizability of MR-based findings of structural volumetric changes and neuroplasticity in the adult human brain. Despite decades of scientific evidence for dynamic interactions between the endocrine and nervous systems, neuroscientific research has largely ignored how endogenous ovarian hormone fluctuations influence human female brain structural plasticity. The current underrepresentation of female samples61,94,95 and female-health variables, such as ovarian hormones, directly limits opportunities for basic scientific discovery and the diversity of human brain health. Our findings suggest that the MTL region has a remarkable degree of plasticity in response to subtle hormone changes during the reproductive years, and that these changes are detectable at the subregion level. Using ultra-high field neuroimaging, this study shows how endogenous hormone fluctuations rapidly and transiently alter volume across the MTL complex in within-subject measurements in multiple participants. We demonstrate the feasibility of a longitudinal MRI design for creating dynamic and personalized maps of the human brain to inform more individualized strategies for neuroimaging the MTL, which may ultimately lead to better understanding of the manifestation and treatment of hormone-related neuropsychiatric and neurodegenerative diseases.
Methods
Participants
Between 15 May 2015 and 21 February 2019, 163 participants were screened, of which 43 could be enrolled in this neuroimaging study in Leipzig (Fig. 3). Participants were financially compensated for study participation. Eligible individuals were female, right-handed, 18–35 years old, with a BMI of 18.5–29 kg m−2, and without any neurological or psychiatric illness (as confirmed with a structured clinical interview and consultation from a licensed psychiatrist, J.S.)62,96. Exclusion criteria were prescription medication or supplement use, tobacco use, positive drug or pregnancy tests, current use or use of hormonal contraceptives within the past six months, current or past hormone therapy, or having been pregnant, postpartum, breastfeeding, or had an abortion within one year of the study. Participants were screened and excluded for DSM-IV axis I disorders and axis II disorders, as well as for the presence of premenstrual mood symptoms using the Premenstrual Symptoms Screening Tool (PSST)97. All participants provided written informed consent after all procedures were fully explained. Forty-one participants were enrolled, of whom two were excluded due to inability to tolerate the 7-T MRI scan, eight voluntarily discontinued because of the time demands of the study, and four were excluded due to irregular cycles, irregularities in bloodwork, or emergency contraceptive pill use after enrollment (included participants N = 27). Of the included participants, 20 completed all six timepoints, two completed five timepoints, one completed three timepoints, one completed two timepoints, and three completed one timepoint (dropout reasons: five due to scheduling conflicts, one used the emergency contraceptive pill, one got an MRI-incompatible retainer), for a total of 138 assessments. The study was conducted in accordance with the Declaration of Helsinki. The Ethical Committee at the Medical Faculty of Leipzig University approved the study, protocol and informed consent forms (#077-11-07032011). The study has been pre-registered at the Open Science Framework (https://osf.io/8mk74/) and is registered on the German Clinical Trials Register (DRKS00030983).
Note that participants may fall into more than one screening exclusion category. *Of the assessed participants, 20 completed all six assessments, two completed five assessments, one completed three assessments, one completed two assessments, and three completed one assessment (dropout reasons: five due to scheduling conflicts, one used the emergency contraceptive pill, one got an MRI-incompatible retainer). PMS, premenstrual syndrome; PMDD, premenstrual dysphoric disorder.
Assessment timing
Participants had a documented history of regular menstrual cycles. In this longitudinal study, assessments occurred during six cycle phases: menstrual (<5 days menses onset), pre-ovulatory (≤2 days before ovulation), ovulation (≤24 h of ovulation), post-ovulatory (≤2 days after ovulation), mid-luteal (6–8 days after ovulation) and premenstrual (≤3 days next menses onset). We developed a systematic protocol for rigorous menstrual cycle monitoring and characterization to determine cycle phase timing as follows. After enrollment, participants used an online application (https://www.mynfp.de) to record daily vaginal basal body temperature, menses information, and cycle day and length information62. Participants recorded menstrual cycle information for at least one full cycle before participating in the six assessments, to provide more information on cycle regularity and length. To determine ovulatory timing, participants underwent multiple vaginal ultrasounds to track growing follicle and detect ovulation, completed luteinizing hormone (LH) urine tests throughout the ovulatory week, and consulted with a gynecologist62. To determine premenstrual timing, we estimated the luteal phase to last ~14 days, supplemented with information from previous menstrual cycle lengths, ovulatory timing and consultation with a gynecologist. The first assessment phase was randomized across participants, and all other assessments took place in remaining chronological order (menstrual, pre-ovulatory, ovulation, post-ovulatory, mid-luteal, premenstrual). At every vaginal ultrasound and assessment visit, hormone levels (estradiol, progesterone, LH, follicle-stimulating hormone (FSH)) were assessed in blood and used to confirm the current cycle phase (based on reference ranges provided by ref. 98 and ECLIA, Cobas:Roche) (Fig. 1).
Blood measurements
Serum from fasting-blood samples was collected at every ultrasound and assessment day visit to measure hormones, confirm cycle phase, ensure physical health, and exclude pregnancy or recent drug-intake. Serum was immediately delivered to the hospital laboratory and kept at 5 °C until assayed within 24 h (ref. 62). Estradiol and progesterone concentrations were determined using high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS), and follicle-stimulating hormone and luteinizing hormone concentrations were determined using electrochemiluminescence immunoassay (ECLIA; Roche). Of the 138 assessments, estradiol values for two assessments and progesterone values for one assessment were not included due to pre-analytical error.
7-T MRI acquisition
Anatomical MRI scans were acquired at the Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, using a Siemens Magnetom 7-T system (Siemens Healthineers) and 32-channel head array coil (NOVA Medical), matched for time of day and without caffeine intake. We acquired high-resolution whole-brain T1-weighted images using an MP2RAGE protocol99 (repetition time (TR) = 5,000 ms; inversion time (TI) 1/2 = 900/2,750 ms; echo time (TE) = 2.45 ms; image matrix, 320 × 320 × 240; voxel size, 0.7 mm × 0.7 mm × 0.7 mm; flip angle 1/2 = 5°/3°; parallel imaging using GRAPPA with acceleration factor = 2). We acquired T2-weighted imaging slabs perpendicular to the anterior–posterior axis of the hippocampus using a Turbo-Spin Echo Sequence (TR = 16,000 ms; TE = 14 ms; image matrix, 384 × 384; 50 slices; voxel size, 0.5 mm × 0.5 mm × 1 mm; refocusing flip angle = 120°; turbo factor = 8; parallel imaging using GRAPPA with acceleration factor = 2).
To minimize motion during the scan, the head, neck and shoulders were secured with foam cushions and the legs were secured with sandbag cushions on the sides. Participants were covered with a blanket to minimize motion of arms and legs, and they were verbally instructed and continuously reminded to remain as still as possible. All incoming images were visually assessed and immediately re-run if motion was detected (this occurred in nine scanning sessions).
MTL segmentation and volumetry
Background noise removal from uniform T1-weighted MP2RAGE image volumes was done using https://github.com/JosePMarques/MP2RAGE-related-scripts. The high-resolution T1- and T2-weighted images were then submitted to the ASHS package35 using the Magdeburg Young Adult 7T Atlas (based on 22 healthy participants; 19–32-years old; 12 females)34, which has been shown to be more sensitive to individual differences in MTL subregion morphology compared to FreeSurfer59. ASHS segmentation software uses a fully automated framework at all stages (MRI pre-processing, rigid body transformation alignment of T1- and T2-weighted images, bias correction and refining, and so on), automatically segmenting the MTL in the T2-weighted MRI scans. To correct for potential motion between the T1 and T2 scans, ASHS uses both FLIRT and ANTS rigid registration algorithms, using the normalized mutual information (NMI) metric as the similarity measure. The method that yields the largest NMI value between the two modalities is used subsequently (full details are available in ref. 35). ASHS documentation, atlases and software are available at https://sites.google.com/view/ashs-dox/ and https://www.nitrc.org/projects/ashs, with technical details and reliability described further in ref. 35. We performed segmentation and bilateral volume calculations for hippocampal (CA1, CA2, CA3, subiculum, dentate gyrus) and adjacent MTL subregions (entorhinal cortex, parahippocampal cortex, perirhinal cortex (segmented into areas 35 and 36)). ITK-SNAP (v3.8) was used for quality assurance (for example, proper alignment of T1- and T2-weighted images). We did not perform manual slice number correction or segmentations, as ASHS is an automatic segmentation tool, and we wanted to best capture anatomical variability and limit idiosyncrasies and researcher degrees of freedom across the segmentation protocols. Quality assurance images were visually assessed by two raters blinded to cycle phase. To assess the reliability of segmentations, the Sørensen-Dice similarity coefficient (0.983) and intraclass correlation coefficient (0.976) were calculated across all assessments in MATLAB, suggesting robust reliability in segmentation. All code is publicly available at https://github.com/RGZsido/MTLPlasticity2022.
Total brain volume, precentral gyrus volume, CSF and CBF
Total brain volume, bilateral precentral gyrus volume and CSF were calculated using the 7-T T1-weighted images and the Segment Data module in the CAT12 toolbox of SPM12 MATLAB R2021a. Image quality was assessed using the overall image quality (image quality ratings, IQR; >91%) as well as noise (>91%), bias (>91%) and image resolution (>91%) parameters. Motion in the T1-weighted images was additionally assessed by detecting ghosting associated with head motion100 (the ghosting ratio should not exceed three). Our values (mean ± s.d., 1.1 ± 0.6) suggest that the overall motion was negligible. Precentral gyrus volume was calculated using the Neuromorphometrics atlas. CBF was measured using a pulsed arterial spin labeling (pASL) sequence (TR = 3,000, TI1 = 700 ms, T1S = 1,775, TI2 = 1,800 ms, TE = 13 ms, flip angle = 90°, matrix size = 64 × 64, slices = 24, field of view (FOV) = 192 × 192 mm, voxel size = 3 × 3 × 4 mm3; labeling slab thickness = 100 mm with a gap of 22 mm, 50 pairs of label and control images), acquired on the same assessment days on a 3-T Magnetom Verio scanner (Siemens) using a 32-channel head coil. The pASL data were preprocessed using an in-house MATLAB analysis pipeline, which included co-registration to the MPRAGE image, motion correction with linear regression, normalization to MNI space using a T1-weighted MPRAGE (TR = 2,300 ms, TI = 900 ms, TE = 4.21 ms, flip angle = 9°, FOV = 256 × 256 mm, slices = 176, bandwidth = 240 Hz px−1, voxel size = 1 × 1 × 1 mm3), and smoothing with a two-dimensional (2D) spatial Gaussian filter with full-width at half-maximum of 3 mm. The CBF values used for the analysis were calculated by pairwise subtraction of labeled and control images by the perfusion model101, as described in more detail in ref. 102.
For the CBF analysis, an anatomical region of interest (ROI) was created as a binary mask of the hippocampus using the WFU PickAtlas toolbox. The mask was resampled to a 3 × 3 × 4-mm voxel size to match the pASL images using the coregister:reslice function in SPM12. The preprocessed CBF maps were multiplied with the binary mask of the hippocampus and the average CBF value (over all voxels within the ROI) was extracted. This was done for all timepoints for each participant.
Statistical analysis
Assuming a medium effect size (η2 = 0.06), an α coefficient of 0.05 and power of 80%, we calculated a total sample size of N = 18 (a priori power analysis, G*Power). In the project protocol, we stated that we aimed to include N = 20 healthy participants with all six timepoints. Estradiol and progesterone values were log-transformed prior to analyses. For outlier detection in brain volumes, we flagged bilateral volumes that were 3 s.d. from the mean for anatomical inspection by two raters. If, on inspection, the volume segmentation maps were unanimously deemed anatomically sound, the volumes were kept to capture reasonable anatomical variation. Of the 138 assessments, we flagged a total of four brain volumes, of which two were determined to be of poor segmentation quality (both for CA1) and were thus removed from further analyses. Bilateral subregion volumes were then adjusted for total brain volume (unstandardized residuals).
For control analyses, we performed linear mixed-effects modeling using the maximum likelihood method of the ‘lmer’ function in the ‘lme4’ R package (v3.5.2) to assess potential effects of hormones (estradiol and progesterone) on CSF, CBF and precentral gyrus volumes. For the main analyses, we used linear mixed-effects models to assess the fixed effects of hormones as well as their interaction with the whole hippocampus and each subregion volume. Inclusion of the interaction term was assessed by comparing model fits using the ‘anova’ function. The P values of the model parameters were calculated via Wald tests and corrected for multiple comparisons using the Benjamini–Hochberg procedure103 controlling for FDR, and were accepted at an FDR-corrected threshold of q < 0.05. For brain volumes that showed significant effects of hormones, we then investigated the fixed effects of cycle phase in another mixed model where timepoint was an independent regressor of subfield volumes. We performed post hoc tests using the ‘difflsmeans’ function and the Satterthwaite correction for degrees of freedom. Participants were included as a random factor in all models. The R code for analyses is publicly available at https://github.com/RGZsido/MTLPlasticity2022.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this Article.
Data availability
Study preregistration is publicly available at https://osf.io/8mk74/. ASHS documentation, atlases and software, as well as the IKND Magdeburg Young Adult 7T Atlas, are available at https://sites.google.com/view/ashs-dox/ and https://www.nitrc.org/projects/ashs. Data are publicly available on an open data repository (https://doi.org/10.17617/3.2CPISW).
Code availability
All code for analyses is publicly available at https://github.com/RGZsido/MTLPlasticity2022.
References
Barha, C. K. & Galea, L. A. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim. Biophys. Acta 1800, 1056–1067 (2010).
Woolley, C. S. & McEwen, B. S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336, 293–306 (1993).
Hao, J. et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 26, 2571–2578 (2006).
Woolley, C. S. & McEwen, B. S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 12, 2549–2554 (1992).
MacLusky, N. J., Luine, V. N., Hajszan, T. & Leranth, C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146, 287–293 (2005).
Hara, Y. et al. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl Acad. Sci. USA 111, 486–491 (2014).
Hara, Y., Waters, E. M., McEwen, B. S. & Morrison, J. H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 95, 785–807 (2015).
Scharfman, H. E. & MacLusky, N. J. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology 76, 696–708 (2014).
Stone, D. J., Rozovsky, I., Morgan, T. E., Anderson, C. P. & Finch, C. E. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. J. Neurosci. 18, 3180–3185 (1998).
Morse, J. K., Scheff, S. W. & DeKosky, S. T. Gonadal steroids influence axon sprouting in the hippocampal dentate gyrus: a sexually dimorphic response. Exp. Neurol. 94, 649–658 (1986).
Arevalo, M.-A., Santos-Galindo, M., Bellini, M.-J., Azcoitia, I. & Garcia-Segura, L. M. Actions of estrogens on glial cells: implications for neuroprotection. Biochim. Biophys. Acta 1800, 1106–1112 (2010).
Stricker, R. et al. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT® analyzer. Clin. Chem. Lab. Med. 44, 883–887 (2006).
Pritschet, L. et al. Functional reorganization of brain networks across the human menstrual cycle. Neuroimage 220, 117091 (2020).
Sundström-Poromaa, I. & Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front. Neurosci. 8, 380 (2014).
Weis, S., Hodgetts, S. & Hausmann, M. Sex differences and menstrual cycle effects in cognitive and sensory resting state networks. Brain Cogn. 131, 66–73 (2019).
Chechko, N. et al. The expectant brain—pregnancy leads to changes in brain morphology in the early postpartum period. Cereb. Cortex 32, 4025–4038 (2022).
Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 (2017).
Luders, E. et al. Gray matter increases within subregions of the hippocampal complex after pregnancy. Brain Imag. Behav. 15, 2790–2794 (2021).
Pawluski, J. L., Brummelte, S., Barha, C. K., Crozier, T. M. & Galea, L. A. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front. Neuroendocrinol. 30, 343–357 (2009).
de Lange, A. M. G. et al. The maternal brain: region‐specific patterns of brain aging are traceable decades after childbirth. Hum. Brain Mapp. 41, 4718–4729 (2020).
Lisofsky, N. et al. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage 118, 154–162 (2015).
Protopopescu, X. et al. Hippocampal structural changes across the menstrual cycle. Hippocampus 18, 985–988 (2008).
Pletzer, B. et al. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 1348, 55–62 (2010).
Frick, K. M. & Kim, J. Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm. Behav. 104, 100–110 (2018).
Schumacher, A. et al. Ventral hippocampal CA1 and CA3 differentially mediate learned approach-avoidance conflict processing. Curr. Biol. 28, 1318–1324 (2018).
González, M. et al. Distribution patterns of estrogen receptor α and β in the human cortex and hippocampus during development and adulthood. J. Comp. Neurol. 503, 790–802 (2007).
Brinton, R. D. et al. Progesterone receptors: form and function in brain. Front. Neuroendocrinol. 29, 313–339 (2008).
Barth, C. et al. In-vivo dynamics of the human hippocampus across the menstrual cycle. Sci. Rep. 6, 32833 (2016).
Arélin, K. et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle—a pilot resting state MRI study. Front. Neurosci. 9, 44 (2015).
Pritschet, L., Taylor, C. M., Santander, T. & Jacobs, E. G. Applying dense-sampling methods to reveal dynamic endocrine modulation of the nervous system. Curr. Opin. Behav. Sci. 40, 72–78 (2021).
Mueller, J. M. et al. Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle. Netw. Neurosci. 5, 125–144 (2021).
Fitzgerald, M., Pritschet, L., Santander, T., Grafton, S. T. & Jacobs, E. G. Cerebellar network organization across the human menstrual cycle. Sci. Rep. 10, 20732 (2020).
Taylor, C. M. et al. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. Neuroimage 220, 117125 (2020).
Berron, D. et al. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage Clin. 15, 466–482 (2017).
Yushkevich, P. A. et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 36, 258–287 (2015).
Ding, S. L. & Van Hoesen, G. W. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto‐and chemoarchitecture. J. Comp. Neurol. 523, 2233–2253 (2015).
Duvernoy, H. M., Cattin, F. & Risold, P.-Y. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI (Springer, 2005).
Amunts, K. et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 210, 343–352 (2005).
Malykhin, N. V., Huang, Y., Hrybouski, S. & Olsen, F. Differential vulnerability of hippocampal subfields and anteroposterior hippocampal subregions in healthy cognitive aging. Neurobiol. Aging 59, 121–134 (2017).
de Flores, R. et al. Effects of age and Alzheimer’s disease on hippocampal subfields: comparison between manual and FreeSurfer volumetry. Hum. Brain Mapp. 36, 463–474 (2015).
Squire, L. R., Stark, C. E. & Clark, R. E. The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004).
Godsil, B. P., Kiss, J. P., Spedding, M. & Jay, T. M. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur. Neuropsychopharmacol. 23, 1165–1181 (2013).
Mitterling, K. L. et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 518, 2729–2743 (2010).
Gervais, N. J. et al. Scene memory and hippocampal volume in middle-aged women with early hormone loss. Neurobiol. Aging 117, 97–106 (2022).
Schlichting, M. L., Zeithamova, D. & Preston, A. R. CA1 subfield contributions to memory integration and inference. Hippocampus 24, 1248–1260 (2014).
West, M. J., Coleman, P. D., Flood, D. G. & Troncoso, J. C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344, 769–772 (1994).
Bali, N. et al. Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology 153, 759–769 (2012).
Hao, J. et al. Estrogen increases the number of spinophilin‐immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 465, 540–550 (2003).
Choi, J. M. et al. Estradiol increases pre-and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta). Endocrinology 144, 4734–4738 (2003).
2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789 (2022).
Berron, D. et al. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain 144, 2771–2783 (2021).
Krumm, S. et al. Cortical thinning of parahippocampal subregions in very early Alzheimer’s disease. Neurobiol. Aging 38, 188–196 (2016).
Olsen, R. K. et al. Human anterolateral entorhinal cortex volumes are associated with cognitive decline in aging prior to clinical diagnosis. Neurobiol. Aging 57, 195–205 (2017).
Ding, S. L., Van Hoesen, G. W., Cassell, M. D. & Poremba, A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic and pathological markers. J. Comp. Neurol. 514, 595–623 (2009).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82, 239–259 (1991).
Buckley, R. F. Menopause status moderates sex differences in tau burden: a Framingham PET Study. Ann. Neurol. 92, 11–22 (2022).
Brinton, R. D., Yao, J., Yin, F., Mack, W. J. & Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 11, 393–405 (2015).
Lee, B. H., Puri, T. A. & Galea, L. A. Sex and sex hormone differences in hippocampal neurogenesis and their relevance to Alzheimer’s disease. In Sex and Gender Differences in Alzheimer’s Disease (eds Ferretti, M. T., Dimech, A. S. & Chadha, A. S.) 23–77 (Academic Press, 2021).
Sone, D. et al. Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high-resolution T2-weighed MR imaging. NeuroImage Clin. 12, 57–64 (2016).
Schmalenberger, K. M. et al. How to study the menstrual cycle: practical tools and recommendations. Psychoneuroendocrinology 123, 104895 (2021).
Taylor, C. M., Pritschet, L. & Jacobs, E. G. The scientific body of knowledge—whose body does it serve? A spotlight on oral contraceptives and women’s health factors in neuroimaging. Front. Neuroendocrinol. 60, 100874 (2021).
Sacher, J. et al. Increase in serotonin transporter binding in patients with premenstrual dysphoric disorder across the menstrual cycle: a case-control longitudinal neuroreceptor ligand positron emission tomography imaging study. Biol. Psychiatry 93, 1081–1088 (2023).
Georgakis, M. K., Beskou-Kontou, T., Theodoridis, I., Skalkidou, A. & Petridou, E. T. Surgical menopause in association with cognitive function and risk of dementia: a systematic review and meta-analysis. Psychoneuroendocrinology 106, 9–19 (2019).
Rice, M. M. et al. Postmenopausal estrogen and estrogen-progestin use and 2-year rate of cognitive change in a cohort of older Japanese American women: the Kame Project. Arch. Intern. Med. 160, 1641–1649 (2000).
Jacobs, D. M. et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology 50, 368–373 (1998).
Sherwin, B. B. Estrogen effects on cognition in menopausal women. Neurology 48, 21S–26S (1997).
Berent-Spillson, A. et al. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology 59, 25–36 (2015).
Henderson, V. Progesterone and human cognition. Climacteric 21, 333–340 (2018).
Zeydan, B. et al. Association of bilateral salpingo-oophorectomy before menopause onset with medial temporal lobe neurodegeneration. JAMA Neurol. 76, 95–100 (2019).
Bruce-Keller, A. J. et al. Antiinflammatory effects of estrogen on microglial activation. Endocrinology 141, 3646–3656 (2000).
Mazzucco, C. et al. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience 141, 1793–1800 (2006).
Nagy, A. I., Ormerod, B. K., Mazzucco, C. & Galea, L. A. Estradiol‐induced enhancement in cell proliferation is mediated through estrogen receptors in the dentate gyrus of adult female rats. Drug Dev. Res. 66, 142–149 (2005).
Waters, E. M. et al. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 1290, 1–11 (2009).
Brake, W. G. et al. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology 142, 1284–1289 (2001).
Galea, L. A. et al. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J. Neuroendocrinol. 25, 1039–1061 (2013).
Daniel, J. M. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm. Behav. 63, 231–237 (2013).
Wisse, L. E. et al. Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol. Aging 35, 2039–2045 (2014).
Deecher, D., Andree, T. H., Sloan, D. & Schechter, L. E. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology 33, 3–17 (2008).
Epperson, C. N. et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am. J. Psychiatry 169, 465–475 (2012).
Gavin, N. I. et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 106, 1071–1083 (2005).
Freeman, E. W., Sammel, M. D., Boorman, D. W. & Zhang, R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry 71, 36–43 (2014).
Zorgdrager, A. & De Keyser, J. Premenstrual exacerbations of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 65, 279–280 (1998).
Confavreux, C. et al. Rate of pregnancy-related relapse in multiple sclerosis. N. Engl. J. Med. 339, 285–291 (1998).
Ramagopalan, S. V., Dobson, R., Meier, U. C. & Giovannoni, G. Multiple sclerosis: risk factors, prodromes and potential causal pathways. Lancet Neurol. 9, 727–739 (2010).
Alvergne, A. & Tabor, V. H. Is female health cyclical? Evolutionary perspectives on menstruation. Trends Ecol. Evol. 33, 399–414 (2018).
Travis, S. et al. High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia 53, 233–245 (2014).
Lee, A. C. et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus 15, 782–797 (2005).
Inhoff, M. C. & Ranganath, C. Significance of objects in the perirhinal cortex. Trends Cogn. Sci. 19, 302–303 (2015).
Aminoff, E. M., Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17, 379–390 (2013).
Kessler, R. C. et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105 (2003).
Kessler, R. C., McGonagle, K. A., Swartz, M., Blazer, D. G. & Nelson, C. B. Sex and depression in the National Comorbidity Survey I: lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 29, 85–96 (1993).
Jin, J. & Maren, S. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 9, 170 (2015).
Wang, A. C., Hara, Y., Janssen, W. G., Rapp, P. R. & Morrison, J. H. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J. Neurosci. 30, 12770–12776 (2010).
Will, T. R. et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 4, ENEURO.0278-17.2017 (2017).
Rechlin, R. K., Splinter, T. F., Hodges, T. E., Albert, A. Y. & Galea, L. A. An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat. Commun. 13, 2137 (2022).
Zsido, R. G. et al. One-week escitalopram intake alters the excitation-inhibition balance in the healthy female brain. Hum. Brain Mapp. 43, 1868–1881 (2022).
Steiner, M., Macdougall, M. & Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Women’s Mental Health 6, 203–209 (2003).
Bae, Y. J. et al. Reference intervals of nine steroid hormones over the life-span analyzed by LC-MS/MS: effect of age, gender, puberty and oral contraceptives. J. Steroid Biochem. Mol. Biol. 193, 105409 (2019).
Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271–1281 (2010).
Gedamu, E. L., Collins, D. L. & Arnold, D. L. Automated quality control of brain MR images. J. Magn. Reson. Imaging 28, 308–319 (2008).
Wong, E. C., Buxton, R. B. & Frank, L. R. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn. Reson. Med. 40, 348–355 (1998).
Uhlig, M. et al. Rapid volumetric brain changes after acute psychosocial stress. Neuroimage 265, 119760 (2023).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B (Methodol.) 57, 289–300 (1995).
Acknowledgements
We thank the following colleagues from the Max Planck Institute for Human Cognitive and Brain Sciences: M. Heinrich for assistance with data acquisition, E. Reimer for assistance with motion artifact detection, N. Weiskopf for assistance in 7-T MRI study design and scanning protocols, H. Schmidt Duderstedt for assistance with data visualization, K. Müller for assistance with MRI data pre-processing, and C. Ketscher, H. Neumayer and J. Nemitz for administrative and editing support. Funding was provided by the Joachim Herz Foundation Fellowship (R.G.Z.), the Society in Science, Branco Weiss Fellowship (J.S.), the Brain & Behavior Research Foundation, a National Association for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant (25032, J.S.) and a Minerva Research Group Grant (Max Planck Society, J.S.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
The principal investigator was J.S. The study was conceived and designed by R.G.Z., C.B., A.V. and J.S. Data acquisition was carried out by R.G.Z. and C.B. Data analysis was performed by R.G.Z., A.N.W., B.S. and L.K. Data were interpreted by R.G.Z., A.N.W., C.B., F.B., A.V.W., A.V. and J.S. T.M. and R.T. contributed to data analysis. The figures were created by R.G.Z. The original draft was written by R.G.Z. The manuscript was reviewed and edited by R.G.Z., A.N.W., C.B., B.S., L.K., T.M., R.T., F.B., A.V.W., A.V. and J.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Hannah Ballard, Gillian Einstein, Emily Jacobs and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zsido, R.G., Williams, A.N., Barth, C. et al. Ultra-high-field 7T MRI reveals changes in human medial temporal lobe volume in female adults during menstrual cycle. Nat. Mental Health 1, 761–771 (2023). https://doi.org/10.1038/s44220-023-00125-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00125-w
This article is cited by
-
Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women
npj Women's Health (2024)
-
Spotlighting SHAPERS: sex hormones associated with psychological and endocrine roles
Neuropsychopharmacology (2024)
-
Learning exceptions to category rules varies across the menstrual cycle
Scientific Reports (2023)