Abstract
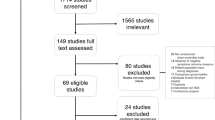
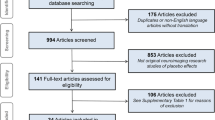
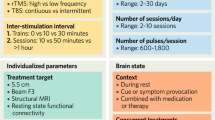
This meta-analysis investigated placebo responses in repetitive transcranial magnetic stimulation treatment for depression, an area with limited systematic analysis. The initial literature search yielded 2,783 relevant records from the past 27 years, leading to the analysis of 52 randomized controlled trials encompassing 54 placebo arms with 2,122 sham participants. Placebo responses were large (d = 1.016) and increasing yearly (Z = 2.18, P = 0.029), irrespective of sham methods, assessment scales or age. Nevertheless, the trial location, number of sites, sample size, sponsor, sex ratio, study quality and medication status had an influence on the active or sham effect and consequently the outcome. Notably, the placebo and active effects increased in parallel (rs = 0.738, P < 0.001), resulting in a time-independent trial outcome. These findings reveal significant placebo responses from 1996 to 2022 but with minimal impact on the trial outcomes, as placebo and active effects demonstrated parallel growth. These results could inform the design of further clinical trials, especially for repetitive transcranial magnetic stimulation in the treatment of depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The meta-analysis is registered on the INPLASY database (INPLASY2022120103) and details can be found at https://inplasy.com/inplasy-2022-12-0103/. All data related to this study are available on the Open Science Framework (OSF) data repository89 at https://osf.io/gczab/?view_only=62c182373b6a49bb89c7fb31c4dfee28.
Code availability
The code used in the current study is publicly available at the OSF repository89 (https://osf.io/gczab/?view_only=62c182373b6a49bb89c7fb31c4dfee28).
References
Cash, R. F. H. et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol. Psychiatry 90, 689–700 (2021).
Malhi, G. S. & Mann, J. J. Depression. Lancet 392, 2299–2312 (2018).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206 (2014).
Mikellides, G., Michael, P. & Tantele, M. Repetitive transcranial magnetic stimulation: an innovative medical therapy. Psychiatriki 32, 67–74 (2021).
Nguyen, K.-H. & Gordon, L. G. Cost-effectiveness of repetitive transcranial magnetic stimulation versus antidepressant therapy for treatment-resistant depression. Value Health 18, 597–604 (2015).
Fitzgibbon, K. P. et al. Cost-utility analysis of electroconvulsive therapy and repetitive transcranial magnetic stimulation for treatment-resistant depression in Ontario. Can. J. Psychiatry 65, 164–173 (2020).
Rossi, S. et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin. Neurophysiol. 132, 269–306 (2021).
Carroll, K. M. in International Encyclopedia of the Social & Behavioral Sciences (eds Smelser, N. J. & Baltes, P. B.) 2043–2048 (Elsevier, 2001).
Hafliðadóttir, S. H. et al. Placebo response and effect in randomized clinical trials: meta-research with focus on contextual effects. Trials 22, 493 (2021).
Moerman, D. E. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med. Anthropol. Q. 14, 51–72 (2000).
Kaptchuk, T. J. & Miller, F. G. Placebo effects in medicine. N. Engl. J. Med. 373, 8–9 (2015).
Walsh, B. T., Seidman, S. N., Sysko, R. & Gould, M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287, 1840–1847 (2002).
Khan, A., Fahl Mar, K., Faucett, J., Khan Schilling, S. & Brown, W. A. Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987–2013. World Psychiatry 16, 181–192 (2017).
Granato, A. et al. Dramatic placebo effect of high frequency repetitive TMS in treatment of chronic migraine and medication overuse headache. J. Clin. Neurosci. 60, 96–100 (2019).
Zebenholzer, K., Thamer, M. & Wober, C. Quality of life, depression, and anxiety 6 months after inpatient withdrawal in patients with medication overuse headache: an observational study. Clin. J. Pain 28, 284–290 (2012).
Benedetti, F., Carlino, E. & Pollo, A. How placebos change the patient’s brain. Neuropsychopharmacology 36, 339–354 (2011).
Bartlett, J., Upshaw, W. N. & Obregon, D. Interactions of the placebo effect and transcranial magnetic stimulation. Prim. Care Companion CNS Disord. https://doi.org/10.4088/PCC.20lr02874 (2021).
Burke, M. J. et al. Placebo effects and neuromodulation for depression: a meta-analysis and evaluation of shared mechanisms. Mol. Psychiatry 27, 1658–1666 (2022).
Schweinhardt, P., Seminowicz, D. A., Jaeger, E., Duncan, G. H. & Bushnell, M. C. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J. Neurosci. 29, 4882–4887 (2009).
Benedetti, F. Neurobiological mechanisms of the placebo effect. J. Neurosci. 25, 10390–10402 (2005).
Brunoni, A. R., Lopes, M., Kaptchuk, T. J. & Fregni, F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS ONE 4, e4824 (2009).
Razza, L. B. et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 105–113 (2018).
Theleritis, C. et al. Two versus one high-frequency repetitive transcranial magnetic stimulation session per day for treatment-resistant depression. J. ECT 33, 190–197 (2017).
Armas-Castañeda, G. et al. Two rTMS sessions per week: a practical approach for treating major depressive disorder. Neuroreport 32, 1364–1369 (2021).
Tong, J. et al. Impact of repetitive transcranial magnetic stimulation (rTMS) on theory of mind and executive function in major depressive disorder and its correlation with brain-derived neurotrophic factor (BDNF): a randomized, double-blind, sham-controlled trial. Brain Sci. https://doi.org/10.3390/brainsci11060765 (2021).
Wang, Y.-M. et al. Randomized controlled trial of repetitive transcranial magnetic stimulation combined with paroxetine for the treatment of patients with first-episode major depressive disorder. Psychiatry Res. 254, 18–23 (2017).
Matsuda, Y., Kito, S., Igarashi, Y. & Shigeta, M. Efficacy and safety of deep transcranial magnetic stimulation in office workers with treatment-resistant depression: a randomized, double-blind, sham-controlled trial. Neuropsychobiology 79, 208–213 (2020).
Ray, S. et al. Efficacy of adjunctive high frequency repetitive transcranial magnetic stimulation of left prefrontal cortex in depression: a randomized sham controlled study. J. Affect. Disord. 128, 153–159 (2011).
Yesavage, J. A. et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry 75, 884–893 (2018).
Carvalho, A. F. et al. Bias in peripheral depression biomarkers. Psychother. Psychosom. 85, 81–90 (2016).
Colloca, L. & Barsky, A. J. Placebo and nocebo effects. N. Engl. J. Med. 382, 554–561 (2020).
Kienle, G. S. & Kiene, H. The powerful placebo effect: fact or fiction? J. Clin. Epidemiol. 50, 1311–1318 (1997).
Kaptchuk, T. J. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann. Intern. Med. 136, 817–825 (2002).
Evers, A. W. M. et al. Implications of placebo and nocebo effects for clinical practice: expert consensus. Psychother. Psychosom. 87, 204–210 (2018).
Liu, A. et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 9, 5092 (2018).
Rief, W. et al. Meta-analysis of the placebo response in antidepressant trials. J. Affect. Disord. 118, 1–8 (2009).
Burke, M. J., Kaptchuk, T. J. & Pascual-Leone, A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann. Neurol. 85, 12–20 (2019).
Kaptchuk, T. J., Goldman, P., Stone, D. A. & Stason, W. B. Do medical devices have enhanced placebo effects? J. Clin. Epidemiol. 53, 786–792 (2000).
Jones, B. D. M. et al. Magnitude of the placebo response across treatment modalities used for treatment-resistant depression in adults: a systematic review and meta-analysis. JAMA Netw. Open 4, e2125531 (2021).
Rodrigues, F. B. & Ferreira, J. J. in International Review of Neurobiology Vol. 153 (eds Witek, N. P. et al.) 49–70 (Academic, 2020).
Davis, N. J., Gold, E., Pascual-Leone, A. & Bracewell, R. M. Challenges of proper placebo control for non-invasive brain stimulation in clinical and experimental applications. Eur. J. Neurosci. 38, 2973–2977 (2013).
Girach, A., Aamir, A. & Zis, P. The neurobiology under the placebo effect. Drugs Today 55, 469–476 (2019).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 (2010).
Rutherford, B. R. & Roose, S. P. A model of placebo response in antidepressant clinical trials. Am. J. Psychiatry 170, 723–733 (2013).
Coleshill, M. J., Sharpe, L., Colloca, L., Zachariae, R. & Colagiuri, B. Placebo and active treatment additivity in placebo analgesia: research to date and future directions. Int. Rev. Neurobiol. 139, 407–441 (2018).
Agid, O. et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am. J. Psychiatry 170, 1335–1344 (2013).
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. & The Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039 (2009).
Cosmo, C., Zandvakili, A., Petrosino, N. J., Berlow, Y. A. & Philip, N. S. Repetitive transcranial magnetic stimulation for treatment-resistant depression: recent critical advances in patient care. Curr. Treat. Options Psychiatry 8, 47–63 (2021).
Givens, G. H., Smith, D. D. & Tweedie, R. L. Publication bias in meta-analysis: a Bayesian data-augmentation approach to account for issues exemplified in the passive smoking debate. Stat. Sci. https://doi.org/10.1214/ss/1030037958 (1997).
Slavin, R. & Smith, D. The relationship between sample sizes and effect sizes in systematic reviews in education. Educ. Eval. Policy Anal. 31, 500–506 (2009).
Egger, M., Smith, G. D., Schneider, M., & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Sterne, J. A. C., Gavaghan, D. & Egger, M. Publication and related bias in meta-analysis. J. Clin. Epidemiol. 53, 1119–1129 (2000).
Rhodes, S. Publication bias in meta-analysis by H. R. Rothstein, A. J. Sutton and M. Borenstein (eds). J. R. Stat. Soc. Ser. A Stat. Soc. 169, 1012–1012 (2006).
Kjaergard, L. L., Villumsen, J. & Gluud, C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann. Intern. Med. 135, 982–989 (2001).
Krzywinski, M. & Altman, N. Power and sample size. Nat. Methods 10, 1139–1140 (2013).
Arias-de la Torre, J. et al. Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. Lancet Public Health 6, e729–e738 (2021).
Koyanagi, A., Oh, H., Stubbs, B., Haro, J. M. & DeVylder, J. E. Epidemiology of depression with psychotic experiences and its association with chronic physical conditions in 47 low- and middle-income countries. Psychol. Med. 47, 531–542 (2017).
Giannakopoulou, O. et al. The genetic architecture of depression in individuals of East Asian ancestry: a genome-wide association study. JAMA Psychiatry 78, 1258–1269 (2021).
Weimer, K., Colloca, L. & Enck, P. Age and sex as moderators of the placebo response – an evaluation of systematic reviews and meta-analyses across medicine. Gerontology 61, 97–108 (2015).
Enck, P. & Klosterhalfen, S. Does sex/gender play a role in placebo and nocebo effects? Conflicting evidence from clinical trials and experimental studies. Front. Neurosci. 13, 160 (2019).
Enck, P., Benedetti, F. & Schedlowski, M. New insights into the placebo and nocebo responses. Neuron 59, 195–206 (2008).
Rubinow, D. R. Sex, drugs, and the neurobiology of the placebo effect. Biol. Psychiatry 79, 788–789 (2016).
Ashar, Y. K., Chang, L. J. & Wager, T. D. Brain mechanisms of the placebo effect: an affective appraisal account. Annu. Rev. Clin. Psychol. 13, 73–98 (2017).
Wang, R. S. et al. Network analysis of the genomic basis of the placebo effect. JCI Insight https://doi.org/10.1172/jci.insight.93911 (2017).
Franconi, F., Campesi, I., Colombo, D. & Antonini, P. Sex–gender variable: methodological recommendations for increasing scientific value of clinical studies. Cells https://doi.org/10.3390/cells8050476 (2019).
Shansky, R. M. & Murphy, A. Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 24, 457–464 (2021).
Shafir, R., Olson, E. & Colloca, L. The neglect of sex: a call to action for including sex as a biological variable in placebo and nocebo research. Contemp. Clin. Trials 116, 106734 (2022).
Merenstein, J. L. & Bennett, I. J. Bridging patterns of neurocognitive aging across the older adult lifespan. Neurosci. Biobehav. Rev. 135, 104594 (2022).
Giedd, J. N. et al. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 40, 43–49 (2015).
Shields, J., Mock, J., Devier, D. & Foundas, A. Unilateral repetitive transcranial magnetic stimulation differentially affects younger and older adults completing a verbal working memory task. J. Neurol. Sci. 384, 15–20 (2018).
Alawi, M., Lee, P. F., Deng, Z.-D., Goh, Y. K. & Croarkin, P. E. Modelling the differential effects of age on transcranial magnetic stimulation induced electric fields. J. Neural Eng. https://doi.org/10.1088/1741-2552/ac9a76 (2023).
Hsu, C.-W., Wang, L.-J. & Lin, P.-Y. Efficacy of repetitive transcranial magnetic stimulation for Tourette syndrome: a systematic review and meta-analysis. Brain Stimul. 11, 1110–1118 (2018).
Goldman, P., Pedersen, E., Bailey, M., Hasse, M. & Koo, M. Age as a determinant of transcranial magnetic stimulation efficacy for major depressive disorder in a naturalistic clinic setting. Brain Stimul. 15, 695–696 (2022).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th edn (American Psychiatric Association Publishing, 2013).
The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (World Health Organization, 1992).
Hackshaw, A. Small studies: strengths and limitations. Eur. Respir. J. 32, 1141–1143 (2008).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn (Routledge, 1988); https://doi.org/10.4324/9780203771587.
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
Montgomery, S. A. & Åsberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389 (1979).
Higgins, J. P. T. & Green, S. (eds) Cochrane Handbook for Systematic Reviews of Interventions 1st edn (Wiley, 2008).
Jadad, A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17, 1–12 (1996).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986).
Hartmann, A., Herzog, T. & Drinkmann, A. Psychotherapy of bulimia nervosa: what is effective? A meta-analysis. J. Psychosom. Res. 36, 159–167 (1992).
Becker, B. J. Synthesizing standardized mean-change measures. Br. J. Math. Stat. Psychol. 41, 257–278 (1988).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Edinger, J. D. & Sampson, W. S. A primary care ‘friendly’ cognitive behavioral insomnia therapy. Sleep 26, 177–182 (2003).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B 57, 289–300 (1995).
Xu, Y.-t. Placebo Response in Transcranial Magnetic Stimulation Treatment for Depression (OSF, accessed 29 July 2023); https://doi.org/10.17605/OSF.IO/GCZAB
Blumberger, D. M. et al. A randomized double-blind sham-controlled comparison of unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant major depression. World J. Biol. Psychiatry 13, 423–435 (2012).
Bretlau, L. G. et al. Repetitive transcranial magnetic stimulation (rTMS) in combination with escitalopram in patients with treatment-resistant major depression: a double-blind, randomised, sham-controlled trial. Pharmacopsychiatry 41, 41–47 (2008).
Croarkin, P. E. et al. Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial. Neuropsychopharmacology 46, 462–469 (2021).
Duprat, R. et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission? J. Affect. Disord. 200, 6–14 (2016).
Fitzgerald, P. B. et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 60, 1002–1008 (2003).
Fitzgerald, P. B. et al. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am. J. Psychiatry 163, 88–94 (2006).
Fitzgerald, P. B. et al. A double blind randomized trial of unilateral left and bilateral prefrontal cortex transcranial magnetic stimulation in treatment resistant major depression. J. Affect. Disord. 139, 193–198 (2012).
George, M. S. et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry 67, 507–516 (2010).
Huang, M. L. et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust. N. Z. J. Psychiatry 46, 257–264 (2012).
Kaster, T. S. et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology 43, 2231–2238 (2018).
Klein, E. et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch. Gen. Psychiatry 56, 315–320 (1999).
Leuchter, A. F. et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 8, 787–794 (2015).
Li, C.-T. et al. Task-modulated brain activity predicts antidepressant responses of prefrontal repetitive transcranial magnetic stimulation: a randomized sham-control study. Chronic Stress https://doi.org/10.1177/24705470211006855 (2021).
Dai, L. et al. The therapeutic effect of repetitive transcranial magnetic stimulation in elderly depression patients. Medicine 99, e21493 (2020).
Martiny, K., Lunde, M. & Bech, P. Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression. Biol. Psychiatry 68, 163–169 (2010).
He, M., Gu, Z., Wang, X. & Tian, X. Effects of repetitive transcranial magnetic stimulation on hypothalamic–pituitary–adrenal axis of patients with depression. J. Med. Coll. PLA 24, 337–345 (2009).
O’Reardon, J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216 (2007).
Rothärmel, M. et al. The priming effect of repetitive transcranial magnetic stimulation on clinical response to electroconvulsive therapy in treatment-resistant depression: a randomized, double-blind, sham-controlled study. Psychol. Med. https://doi.org/10.1017/S0033291721003810 (2021).
Tavares, D. F. et al. Treatment of mixed depression with theta-burst stimulation (TBS): results from a double-blind, randomized, sham-controlled clinical trial. Neuropsychopharmacology 46, 2257–2265 (2021).
Ullrich, H., Kranaster, L., Sigges, E., Andrich, J. & Sartorius, A. Ultra-high-frequency left prefrontal transcranial magnetic stimulation as augmentation in severely ill patients with depression: a naturalistic sham-controlled, double-blind, randomized trial. Neuropsychobiology 66, 141–148 (2012).
van Belkum, S. M. et al. No antidepressant effects of low intensity transcranial pulsed electromagnetic fields for treatment resistant depression. J. Affect. Disord. 294, 679–685 (2021).
Xie, M., Jiang, W. & Yang, H. Efficacy and safety of the Chinese herbal medicine shuganjieyu with and without adjunctive repetitive transcranial magnetic stimulation (rTMS) for geriatric depression: a randomized controlled trial. Shanghai Arch. Psychiatry 27, 103–110 (2015).
Zavorotnyy, M. et al. Intermittent theta-burst stimulation moderates interaction between increment of N-acetyl-aspartate in anterior cingulate and improvement of unipolar depression. Brain Stimul. 13, 943–952 (2020).
Leuchter, A. et al. Adaptive Design Study of NEST sTMS in Subjects With Major Depressive Disorder https://clinicaltrials.gov/study/NCT03288714 (2021).
Avery, D. H. et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol. Psychiatry 59, 187–194 (2006).
Blumberger, D. M. et al. Unilateral and bilateral MRI-targeted repetitive transcranial magnetic stimulation for treatment-resistant depression: a randomized controlled study. J. Psychiatry Neurosci. 41, E58–E66 (2016).
Carpenter, L. L. et al. rTMS with a two-coil array: safety and efficacy for treatment resistant major depressive disorder. Brain Stimul. 10, 926–933 (2017).
Frick, A., Persson, J. & Bodén, R. Habitual caffeine consumption moderates the antidepressant effect of dorsomedial intermittent theta-burst transcranial magnetic stimulation. J. Psychopharmacol. 35, 1536–1541 (2021).
Herwig, U. et al. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br. J. Psychiatry 191, 441–448 (2007).
Levkovitz, Y. et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 14, 64–73 (2015).
Pan, F. et al. Neuronavigation-guided rTMS for the treatment of depressive patients with suicidal ideation: a double-blind, randomized, sham-controlled trial. Clin. Pharmacol. Ther. 108, 826–832 (2020).
Brunelin, J. et al. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimul. 7, 855–863 (2014).
Chou, P. H. et al. Antidepressant efficacy and immune effects of bilateral theta burst stimulation monotherapy in major depression: a randomized, double-blind, sham-controlled study. Brain Behav. Immun. 88, 144–150 (2020).
Dunlop, K. et al. Dorsomedial prefrontal cortex repetitive transcranial magnetic stimulation for treatment-refractory major depressive disorder: a three-arm, blinded, randomized controlled trial. Brain Stimul. 13, 337–340 (2020).
Pallanti, S., Bernardi, S., Di Rollo, A., Antonini, S. & Quercioli, L. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience 167, 323–328 (2010).
Rumi, D. O. et al. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol. Psychiatry 57, 162–166 (2005).
Zhang, Z. et al. Task-related functional magnetic resonance imaging-based neuronavigation for the treatment of depression by individualized repetitive transcranial magnetic stimulation of the visual cortex. Sci. China Life Sci. 64, 96–106 (2021).
Wang, X. et al. Therapeutic efficacy of connectivity-directed transcranial magnetic stimulation on anticipatory anhedonia. Depress. Anxiety 38, 972–984 (2021).
Struckmann, W., Persson, J., Weigl, W., Gingnell, M. & Bodén, R. Modulation of the prefrontal blood oxygenation response to intermittent theta-burst stimulation in depression: a sham-controlled study with functional near-infrared spectroscopy. World J. Biol. Psychiatry https://doi.org/10.1080/15622975.2020.1785007 (2020).
Koerselman, F., Laman, D. M., van Duijn, H., van Duijn, M. A. J. & Willems, M. A. M. A 3-month, follow-up, randomized, placebo-controlled study of repetitive transcranial magnetic stimulation in depression. J. Clin. Psychiatry 65, 1323–1328 (2004).
Rossini, D. et al. Does rTMS hasten the response to escitalopram, sertraline, or venlafaxine in patients with major depressive disorder? A double-blind, randomized, sham-controlled trial. J. Clin. Psychiatry 66, 1569–1575 (2005).
Yu, F. et al. Repetitive transcranial magnetic stimulation promotes response inhibition in patients with major depression during the stop-signal task. J. Psychiatr. Res. 151, 427–438 (2022).
Pan, F. et al. Effects of neuronavigation-guided rTMS on serum BDNF, TrkB and VGF levels in depressive patients with suicidal ideation. J. Affect. Disord. 323, 617–623 (2023).
Wilkening, J., Witteler, F. & Goya-Maldonado, R. Suicidality and relief of depressive symptoms with intermittent theta burst stimulation in a sham-controlled randomized clinical trial. Acta Psychiatr. Scand. 146, 540–556 (2022).
Dai, L. et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) accelerates onset time of beneficial treating effects and improves clinical symptoms of depression. CNS Neurol. Disord. Drug Targets 21, 500–510 (2022).
Acknowledgments
This study was funded by STI2030-Major Projects (2021ZD0203900), Natural Science Foundation of China (NSFC) grant (32241015, T.-F.Y.; 32071054, Y.T.), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20181715, T.-F.Y.), the Science and Technology Commission of Shanghai Municipality (23XD1423000 and 23ZR1480800, T.-F.Y.) and the Shanghai Municipal Commission of Health (2022JC016, T.-F.Y.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.-F.Y. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. T.-F.Y., Y.T. and Y.X. were responsible for the concept and design. All authors carried out the acquisition, analysis or interpretation of data. Y.X., Y.Z. and D.Z. drafted the paper. D.Z., T.-F.Y. and Y.T. were responsible for critical revision of the paper for important intellectual content. Y.X. and Y.Z. carried out the statistical analysis. T.-F.Y. obtained the funding and provided administrative, technical or material support. Supervision was by T.-F.Y. and Y.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Sujit K. Kar, Tyler Kaster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
PubMed search strategy and Supplementary Tables 1–4 and Figs. 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Zhang, Y., Zhao, D. et al. Growing placebo response in TMS treatment for depression: a meta-analysis of 27-year randomized sham-controlled trials. Nat. Mental Health 1, 792–809 (2023). https://doi.org/10.1038/s44220-023-00118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00118-9