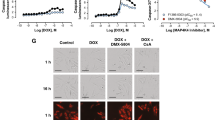
Abstract
Targeting Meis1 and Hoxb13 transcriptional activity could be a viable therapeutic strategy for heart regeneration. In this study, we performd an in silico screening to identify FDA-approved drugs that can inhibit Meis1 and Hoxb13 transcriptional activity based on the resolved crystal structure of Meis1 and Hoxb13 bound to DNA. Paromomycin (Paro) and neomycin (Neo) induced proliferation of neonatal rat ventricular myocytes in vitro and displayed dose-dependent inhibition of Meis1 and Hoxb13 transcriptional activity by luciferase assay and disruption of DNA binding by electromobility shift assay. X-ray crystal structure revealed that both Paro and Neo bind to Meis1 near the Hoxb13-interacting domain. Administration of Paro–Neo combination in adult mice and in pigs after cardiac ischemia/reperfusion injury induced cardiomyocyte proliferation, improved left ventricular systolic function and decreased scar formation. Collectively, we identified FDA-approved drugs with therapeutic potential for induction of heart regeneration in mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings in this study are included in the main article and associated files. Source Data are provided with the manuscript. The crystal structure for the Meis1 homeobox domain bound to paromomycin or neomycin fragment was deposited to the Protein Data Bank with accession codes 8VTS and 8VTT, respectively. ChIP-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE240437. RNA-seq data have been deposited in the GEO under accession number GSE240519.
References
Bui, A. L., Horwich, T. B. & Fonarow, G. C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 8, 30–41 (2011).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Poss, K. D. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45 (2007).
Walsh, S., Ponten, A., Fleischmann, B. K. & Jovinge, S. Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 86, 365–373 (2010).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Soonpaa, M. H., Kim, K. K., Pajak, L., Franklin, M. & Field, L. J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271, H2183–H2189 (1996).
Chaudhry, H. W. et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 279, 35858–35866 (2004).
Soonpaa, M. H. et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J. Clin. Invest. 99, 2644–2654 (1997).
Sim, C. B. et al. Dynamic changes in the cardiac methylome during postnatal development. FASEB J. 29, 1329–1343 (2015).
Trivedi, C. M., Lu, M. M., Wang, Q. & Epstein, J. A. Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J. Biol. Chem. 283, 26484–26489 (2008).
Puente, B. N. et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014).
Porrello, E. R. et al. miR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 109, 670–679 (2011).
Eulalio, A. et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Chen, J. et al. mir-17–92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 112, 1557–1566 (2013).
Kiserud, T. Physiology of the fetal circulation. Semin. Fetal Neonatal Med. 10, 493–503 (2005).
Nguyen, N. U. N. et al. A calcineurin–Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature 582, 271–276 (2020).
Henley, M. J. & Koehler, A. N. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat. Rev. Drug Discov. 20, 669–688 (2021).
Arkin, M. R., Tang, Y. & Wells, J. A. Small-molecule inhibitors of protein–protein interactions: progressing toward the reality. Chem. Biol. 21, 1102–1114 (2014).
Grembecka, J. et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol. 8, 277–284 (2012).
Ahmed, M. S. et al. Identification of tetracycline combinations as EphB1 tyrosine kinase inhibitors for treatment of neuropathic pain. Proc. Natl Acad. Sci. USA 118, e2016265118 (2021).
Yin, Y. et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356, eaaj2239 (2017).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016).
Musa, A. M. et al. Paro for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study. PLoS Negl. Trop. Dis. 4, e855 (2010).
Goeman, F. et al. Molecular imaging of nuclear factor-Y transcriptional activity maps proliferation sites in live animals. Mol. Biol. Cell 23, 1467–1474 (2012).
Kocabas, F. et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 120, 4963–4972 (2012).
Liu, Y. et al. Pharmacokinetics of neomycin sulfate after intravenous and oral administrations in swine. J. Vet. Pharmacol. Ther. 44, 850–853 (2021).
Bruni, G. N. & Kralj, J. M. Membrane voltage dysregulation driven by metabolic dysfunction underlies bactericidal activity of aminoglycosides. eLife 9, e58706 (2020).
Jospe-Kaufman, M., Siomin, L. & Fridman, M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 30, 127218 (2020).
Zeitz, M. J. & Smyth, J. W. Translating translation to mechanisms of cardiac hypertrophy. J. Cardiovasc. Dev. Dis. 7, 9 (2020).
Ye, L. et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761 (2014).
Halgren, T. A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 17, 490–519 (1996).
Naïm, M. et al. Solvated interaction energy (SIE) for scoring protein–ligand binding affinities. 1. Exploring the parameter space. J. Chem. Inf. Model. 47, 122–133 (2007).
Labute, P. Protonate3D: assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 75, 187–205 (2009).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Hara, H. et al. Discovery of a small molecule to increase cardiomyocytes and protect the heart after ischemic injury. JACC Basic Transl. Sci. 3, 639–653 (2018).
Arsic, N. et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol. Ther. 7, 450–459 (2003).
Lam, N. T. et al. Targeting calcineurin induces cardiomyocyte proliferation in adult mice. Nat. Cardiovasc. Res. 1, 679–688 (2022).
Mahmoud, A. I. et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253 (2013).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2013).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–w205 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 66, 1153–1163 (2010).
Acknowledgements
The authors gratefully acknowledge the Molecular Pathology Core at The University of Texas Southwestern Medical Center (UTSW) and J. Shelton for help with histology; J. Xu and Y. J. Kim from the Next Generation Sequencing Core Facility at the Children’s Research Institute at UTSW for performing the Illumina sequencing; and E. Olson, M. Parker, Z. Wang, B. McConnell and the Whole Brain Microscopy Facility at UTSW for their support and assistance in this work. The authors thank the Structural Biology Laboratory and H. Aronovich at UTSW for support with X-ray crystallographic studies. Results shown in this report are derived from work performed at the Argonne National Laboratory and the Structural Biology Center at the Advanced Photon Source, under Department of Energy Office of Biological and Environmental Research contract DE-AC02-06CH11357. H.A.S. was supported by grants from the National Institutes of Health (NIH) (R01 HL137415-02, R01 HL147276-01, R01 HL149137-01, 1P01HL160476-01A1, R35 HL166563-01 and P01HL160488), the Cancer Prevention and Research Institute of Texas (RP160520), the Hamon Center for Regenerative Science and Medicine and the Leducq Foundation (Redox Regulation of Cardiomyocyte Renewal). M.S.A was funded by NIH R01GM119336. N.U.N.N. was supported by grants from the American Heart Association (856552 and 19POST34450039). N.V.G was supported by grants from the NIH (GM127390) and the Welch Foundation (I-1505). C.C.Z. was supported by Leukemia & Lymphoma Society grant (6629-21) and NIH (R01CA248736). Work in the M.G. laboratory was supported by European Research Council Advanced Grant 787971 ‘CuRE’; British Heart Foundation Programme Grant RG/19/11/34633; and grants 825670 ‘CardioReGenix’ and 874764 ‘REANIMA’ from the European Commissionʼs Horizon 2020 program.
Author information
Authors and Affiliations
Contributions
M.S.A. and N.U.N.N. conducted and interpreted experiments related to protein expression and purification and mouse proliferation experiments without myocardial injury. M.S.A. and C.-C.H. performed all mouse injections. N.U.N.N. and C.-C.H. performed mouse surgeries and echocardiography experiments and interpreted the results. M.S.A. and W.E. performed and interpreted non-injury mouse echocardiography. N.U.N.N., C.-C.H. and N.L. conducted mouse proliferation experiments with myocardial injury and interpreted results. N.U.N.N. and N.T.L. performed and interpreted proliferation from dispersed hearts. S.T., M.S.A., C.-C.H. and N.U.N.N. managed mouse colonies. Y.N., G.W., X.L. and J.Z. performed and interpreted the pig-related experiment. M.S.A. and A.F. designed and performed the docking simulations and scoring experiments. N.U.N.N., P.W., D.T. and M.S.A. conducted and interpreted crystallization experiments. J.P. and N.V.G. helped with crystal structure interpretation and figure generation. N.U.N.N., M.S.A., C.-C.H. and N.T.L. conducted immunofluorescent staining. M.S.A., A.F. and I.M.-M. performed and interpreted the NRVM screening and BrdU experiments. N.U.N.N. and M.S.A. conducted MADM-related experiments and interpreted results. N.U.N.N., C.-C.H., M.C. and M.D.S. conducted ChIP-seq. N.U.N.N., C.-C.H. and I.M.-M. conducted RNA-seq. Y.L., N.U.N.N., C.X., E.O., D.Z. and I.M.-M. interpreted ChIP-seq and RNA-seq results. I.S., M.T., L.Z. and M.G. performed and interpreted CycleTrack experiments. X.L. and C.-C.Z. performed and interpreted HSC experiments. N.U.N.N. and C.-C.H. performed and interpreted experiments related to protein purification, cell culture, transfection, western blotting, luciferase assays, EMSA, qPCR and co-IP. M.S.A. and N.U.N.N. designed and conducted experiments, interpreted results and contributed to manuscript preparation. H.A.S. designed the experiments, conceived the project and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
M.G. is founder, consultant, member of the board and equity holder in Forcefield Therapeutics, Heqet Therapeutics and Purespring Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of Paro and Neo via a structure-based drug repurposing.
a, Chemical structures of Neo and Paro. b, Docked poses for Neo (magenta) and Paro (green) against S1-S3 sites for MEIS1-HOXB13 crystal structure. Luciferase transcriptional activity assay for p15 with c, Meis1 and d, Hoxb13 against hesperidin and rutin, compared to DMSO (Ctrl). Statistical analyses: two way ANOVA with Tukey’s post-hoc test (c,d); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 2 Purification and crystallization of Meis1 and Hoxb13.
a-b, Size-exclusion chromatography (Superdex 200) of DBD Meis1 and Hoxb13 purified proteins, respectively. The Y axis shows the absorbance at 280 nm and the X axis shows the elution volume in ml. c-d, Purified and peak fractions of (c) Meis1 and (d) Hoxb13 were analysed by SDS-PAGE and visualised with Coomassie Blue staining. e-f, Quantification for (e) Meis1 and (f) Hoxb13 EMSA showing that Paro, Neo, and Paro–Neo combination disrupt their DNA binding capacities. g, Paro–Neo dissociation constant for Meis1-DNA binding in EMSA assays across 0.001-50 µM drug concentrations. Data are presented as mean ± s.e.m. Statistical analyses: one way ANOVA with Tukey’s post-hoc test (e, f); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 3 Electron density for Paro and Neo bound Meis1.
a, Crystals of Meis1 grown with 10 mM Neo in hanging drop. b, Close-up of Ribostamycin (grey) bound to Meis1 interacting domains showing the contacts of H 297, P 298, and Y 299 as well as the role of E 303 to form hydrogen-bond interactions. c, Electron density for Paro-bound Meis1: Shown in grey mesh is the ½2mFo-DFc½ electron density, contoured at 0.5 s. Shown in stick representation are H 297 (green) in chain A, H 294 (yellow) in chain D and the Ribostamycin fragment of Paro (cyan). d, Electron density for Neo-bound Meis1: shown in Grey mesh is the ½2mFo-DFc½ electron density, contoured at 0.7 s. Shown in stick representation are H 297 (green) in chain G, H 294 (yellow) in the symmetry-related chain D and the Ribostamycin fragment of neomycin (cyan). e, Ribbon cartoon representation for Hoxb13 (Cyan) and Meis1 (Orange) along with Ribostamycin (Purple) and close-up for the contributing amino acid residues from Hoxb13 and Meis1 along with Ribostamycin. This was modelled using alignment of PDB_ID; 5EGO and Paro bound to Meis1 at RMSD 0.373 A°. f, Ribbon cartoon representation for Hoxb13 (Cyan) and Meis1 (Orange) along with Ribostamycin (Purple) and close-up for the contributing amino acid residues from Hoxb13 and Meis1 along with Ribostamycin. This was modelled using alignment of PDB_ID: 5EGO and Neo bound to Meis1 at RMSD 0.373 A°.
Extended Data Fig. 4 Paro treatment promotes adult cardiomyocyte division.
a-c, (a) BW, (b) HW, and (c) HW/BW in control and Paro-treated 10-weeks CD-1 mice. Ctrl, n = 5; Paro-treated groups, n = 5. d, Representative images of immunostaining for pH3 (green), cTnT (red) and nucleus (blue), showing additional examples of mitotic cardiomyocytes in Paro-treated mice. e, Representative images of immunostaining for AurkB (green), cTnT (red) and nucleus (blue), showing additional examples of cytokinesis cardiomyocytes in Paro-treated mice. f, Representative images and quantification for pH3 and cTnT staining in Paro-treated isolated cardiomyocytes. Scale bars, 10 μm (d-f). Data are presented as mean ± s.e.m.; Student’s unpaired two-sided t-test. Data in a-c were done for n = 5 for each group. Data in f were done for n = 4 for each group. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 5 Paro–Neo treatment prolongs neonatal cardiomyocyte proliferation.
a, Schematic for Paro–Neo administration to neonates CD-1 pups at 300 mg kg−1, i.p from p1 to p14. Hearts were collected for histological analysis. b, (Left, upper and right) Representative images of immunostaining for pH3 (green), cTnT (red) and nucleus (blue) showing mitotic cardiomyocytes (arrowheads) in Paro–Neo-treated mice; and (left, lower) the quantification. Ctrl, n = 6; Paro–Neo-treated groups, n = 3. c, (Left, upper and right) Representative images of immunostaining for aurora B kinase (green), cTnT (red) and nucleus (blue) showing cytokinesis cardiomyocytes (arrowheads) in Paro–Neo-treated mice; and (left, lower) the quantification. Ctrl, n = 6; Paro–Neo-treated groups, n = 3. d, Representative images and quantification for CSA cardiomyocyte size from WGA (green) and nucleus (blue) staining show no difference in cell size between Paro–Neo and vehicle-treated hearts. Ctrl, n = 3; Paro–Neo-treated groups, n = 4. Data are presented as mean ± s.e.m.; Student’s unpaired two-sided t-test (b,c). Data in b-d were done for at least n = 3 for each group. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars, 10 μm (b,c) d-f and 100 μm (d).
Extended Data Fig. 6 Paro–Neo treatment promotes adult cardiomyocyte division.
a, Schematic for Paro–Neo administration to adult CD-1 mice at 300 mg kg−1, i.p. for 2 weeks. Hearts were collected for histological analysis. b-d, (b) BW, (c) HW, and (d) HW/BW in control and Paro–Neo-treated mice. e, Representative images of immunostaining for pH3 (green), cTnT (red) and nucleus (blue), showing additional examples of mitotic cardiomyocytes in Paro–Neo-treated mice. f, Representative images of immunostaining for AurkB (green), cTnT (red) and nucleus (blue), showing additional examples of cytokinesis cardiomyocytes (arrowheads) in Paro–Neo-treated mice. g, (Upper) Representative images and (lower) quantification for pH3 and cTnT staining in Paro–Neo-treated isolated cardiomyocytes. Data are presented as mean ± s.e.m.; Student’s unpaired two-sided t-test (b-d, g). Data in b-d were done for n = 5 for control group and n = 8 for Paro–Neo-treated group. Data in g were done for n = 4 for each group. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars, 10 μm (e-g).
Extended Data Fig. 7 RNA-seq with ChIP-seq guided targets.
a, Representative sequencing tracks showing Meis1 ChIP-seq peaks in vehicle-treated (blue) and Paro–Neo-treated (red) hearts at Myh6 locus demonstrating the absence of the peaks in Paro–Neo-treated samples. b, Unbiased RNA-seq (with a fold change of 1.5). Venn diagrams show the common genes of two cohorts (DiKO vs Paro–Neo-treated hearts, normalised to Ctrl). c-d, GO terms of (c) dKO and (d) Paro–Neo-treated hearts.
Extended Data Fig. 8 In vivo pharmacological effect of aminoglycosides on the expression of cell cycle inhibitors, Meis1, Hoxb13, and HSCs.
a, Neonatal CD-1 mice received twice daily injections, i.p. of PBS, Paro (100 mg kg−1), Neo (100 mg kg−1), and Paro–Neo (150 mg kg−1) starting from p14 till p28. b-d, (b) Representative WB and densitometry quantification of (c) p15/p16 and (d) p21 protein expression, n = 4 for each group. Gapdh serves as loading controls. e-f, mRNA expression of (e) Meis1 and (f) Hoxb13 following drug treatment, n = 3 for each group. g-i, (g) Representative WB and densitometry quantification of (h) Meis1 and (i) Hoxb13 protein expression, n = 3 for each group. Gapdh serves as loading controls. j-l, Paro–Neo administration increases HSC number. 10-week-old mice received daily injections of Ctrl or Paro–Neo for 14 days. k, Representative flow cytometry for Ctrl and Paro–Neo-treated samples. l, Histograms of the means of LT-HSCs. Ctrl, n = 8; Paro/Neo-treated groups, n = 7. Data are presented as mean ± s.e.m. Statistical analyses: one-way ANOVA with Tukey’s post-hoc test (c-f, h, i), Student’s unpaired two-sided t-test (l); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 9 Aminoglycosides prevent cardiac remodelling in MI mouse model.
a, Schematic of MI model in drug(s)-treated mice b, Serial echocardiography assessment of LVEF showing maintained LVEF post-MI in Paro- and Paro–Neo-treated mice, compared with controls. However, higher LVEF post-MI in Paro–Neo-treated mice was observed. c, Representative echocardiography images for Ctrl, Paro-, and Paro–Neo-treated hearts at 1 week (pre-injection) and 8 weeks post-MI. d-k, Serial echocardiographic measurement of Ctrl, Paro-, and Paro–Neo-treated mice. l, Masson’s trichrome staining of hearts post-MI shows a marked decrease in LV dilatation and remodelling of Paro–Neo-treated hearts, compared with Ctrl hearts. m, Paro-treated hearts show a non-significant decline in the infarcted size, while Paro–Neo-treated hearts show a significant decrease in the infarcted size midline length, compared with controls. Ctrl, n = 6; Paro, n = 5, and Paro–Neo-treated groups, n = 8. n-p, (n) BW, (o) HW, and (p) HW/BW in Ctrl, Paro-, and Paro–Neo-treated mice. Ctrl, n = 6; Paro, n = 5, and Paro–Neo-treated groups, n = 5. q, Wet to dry lung ratio shows a significant decrease in the Paro and Paro–Neo-treated mice, compared with controls. Ctrl, n = 6; Paro, n = 5, and Paro–Neo-treated groups, n = 5. Data are presented as mean ± s.e.m. Statistical analyses: two-way ANOVA with Tukey’s post-hoc test (b); one-way ANOVA with Tukey’s post-hoc test (m-q); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 10 Schematic of Paro and Neo inhibiting Meis1 and Hoxb13 transcriptional activity.
In-silico screening identifies the FDA-approved drugs Paromomycin and Neomycin as inhibitors of Meis1 and Hoxb13 DNA binding, thereby promoting heart regeneration.
Supplementary information
Supplementary Table 1
Data collection and refinement statistics and Meis1 structures
Source data
Source Data Fig. 1d–f
Statistical source
Source Data Fig. 2a–c
Statistical source
Source Data Fig. 2i
Uncropped western blots
Source Data Fig. 3b–g
Statistical source
Source Data Fig. 4a–f,j,n
Statistical source
Source Data Fig. 6a,c e,f,h–k
Statistical source
Source Data Fig. 7b–i
Statistical source
Source Data Extended Data Fig. 1c,d
Statistical source
Source Data Extended Data Fig. 2d
Uncropped gel
Source Data Extended Data Fig. 2h,g
Statistical source
Source Data Extended Data Fig. 4a–c,f
Statistical source
Source Data Extended Data Fig. 5b–d
Statistical source
Source Data Extended Data Fig. 6b–d,g
Statistical source
Source Data Extended Data Fig. Table 8b
Uncropped western blots
Source Data Extended Data Fig. 8c–i,l
Statistical source
Source Data Extended Data Fig. 9b,d–i
Statistical source
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, M.S., Nguyen, N.U.N., Nakada, Y. et al. Identification of FDA-approved drugs that induce heart regeneration in mammals. Nat Cardiovasc Res 3, 372–388 (2024). https://doi.org/10.1038/s44161-024-00450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00450-y
This article is cited by
-
Toward drug-induced heart regeneration
Nature Cardiovascular Research (2024)