Abstract
Abdominal aortic aneurysm (AAA) is a highly lethal vascular disease. The role of platelets in AAA remains incompletely understood. Here we show that platelet ceramides, rather than other phospholipids, were elevated in an angiotensin II (AngII)-induced AAA murine model and in patients with AAA by using targeted lipidomic analysis. Among key ceramide metabolism enzymes, alkaline ceramidase 1 (Acer1) hydrolyzing ceramides were exclusively downregulated in AAA platelets. Platelet-specific Acer1 knockout mice were more susceptible to AAA upon AngII infusion without affecting hemostasis and thrombosis. Mechanistically, Acer1 deficiency in platelets facilitated platelet pro-inflammatory cytokine secretion as well as P-selectin-mediated circulating platelet–leukocyte aggregation and infiltration in aortic walls via the ceramide–p38 MAPK signaling axis. Of note, AngII repressed Acer1 expression in platelets by decreasing HuR-dependent mRNA stability. In conclusion, Acer1-mediated ceramide degradation in platelets exhibited anti-inflammatory effects and ameliorated AAA formation, potentially serving as a therapeutic target for AAA and other inflammatory vascular diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the analyses presented in this study are provided in the text and its associated files.
References
Daugherty, A. & Cassis, L. A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 24, 429–434 (2004).
Golledge, J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16, 225–242 (2019).
van der Meijden, P. E. J. & Heemskerk, J. W. M. Platelet biology and functions: new concepts and clinical perspectives. Nat. Rev. Cardiol. 16, 166–179 (2019).
Vorp, D. A., Mandarino, W. A., Webster, M. W. & Gorcsan, J. 3rd Potential influence of intraluminal thrombus on abdominal aortic aneurysm as assessed by a new non-invasive method. Cardiovasc. Surg. 4, 732–739 (1996).
Owens, A. P. 3rd et al. Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 35, 2032–2041 (2015).
Umebayashi, R. et al. Cilostazol attenuates angiotensin II-induced abdominal aortic aneurysms but not atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 38, 903–912 (2018).
Lindholt, J. S., Sorensen, H. T., Michel, J. B., Thomsen, H. F. & Henneberg, E. W. Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc. Endovasc. Surg. 42, 329–334 (2008).
Thompson, A. et al. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J. Vasc. Surg. 52, 55–61 (2010).
Rose, J. et al. Comparative safety of endovascular aortic aneurysm repair over open repair using patient safety indicators during adoption. JAMA Surg. 149, 926–932 (2014).
Dolegowska, B., Lubkowska, A. & De Girolamo, L. Platelet lipidomic. J. Biol. Regul. Homeost. Agents 26, 23s–33s (2012).
Laaksonen, R. et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016).
Lemaitre, R. N. et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ. Heart Fail. 12, e005708 (2019).
Chatterjee, M. et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur. Heart J. 38, 1993–2005 (2017).
Folkesson, M. et al. Inflammatory cells, ceramides, and expression of proteases in perivascular adipose tissue adjacent to human abdominal aortic aneurysms. J. Vasc. Surg. 65, 1171–1179 (2017).
Meher, A. K. et al. Novel role of IL (interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 38, 843–853 (2018).
Liao, S. et al. Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J. Vasc. Surg. 33, 1057–1064 (2001).
Mullen, T. D., Hannun, Y. A. & Obeid, L. M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441, 789–802 (2012).
Bishton, M. J. et al. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood 117, 3658–3668 (2011).
Tan, M., Qing, K., Zhou, S., Yoder, M. C. & Srivastava, A. Adeno-associated virus 2-mediated transduction and erythroid lineage-restricted long-term expression of the human β-globin gene in hematopoietic cells from homozygous β-thalassemic mice. Mol Ther 3, 940–946 (2001).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67 (2003).
Zarbock, A., Polanowska-Grabowska, R. K. & Ley, K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 21, 99–111 (2007).
Draper, J. M. et al. Discovery and evaluation of inhibitors of human ceramidase. Mol. Cancer Ther. 10, 2052–2061 (2011).
Yang, M. et al. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 129, 2917–2927 (2017).
Li, Z., Delaney, M. K., O’Brien, K. A. & Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 30, 2341–2349 (2010).
Beiter, T. et al. Exercise, skeletal muscle and inflammation: ARE-binding proteins as key regulators in inflammatory and adaptive networks. Exerc. Immunol. Rev. 21, 42–57 (2015).
Wanhainen, A. et al. The effect of ticagrelor on growth of small abdominal aortic aneurysms—a randomized controlled trial. Cardiovasc. Res. 116, 450–456 (2020).
Tani, M., Sano, T., Ito, M. & Igarashi, Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J. Lipid Res. 46, 2458–2467 (2005).
Bariana, T. K. et al. Sphingolipid dysregulation due to lack of functional KDSR impairs proplatelet formation causing thrombocytopenia. Haematologica 104, 1036–1045 (2019).
Münzer, P. et al. Acid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 34, 61–71 (2014).
Tong, J. & Holzapfel, G. A. Structure, mechanics, and histology of intraluminal thrombi in abdominal aortic aneurysms. Ann. Biomed. Eng. 43, 1488–1501 (2015).
Boilard, E. Platelet-derived interleukin-1β fuels the fire in blood vessels in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 607–608 (2017).
Johnston, W. F. et al. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 33, 294–304 (2013).
Wong, D. J. et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood 138, 1182–1193 (2021).
Wu, G. et al. Complement regulator CD59 protects against angiotensin II-induced abdominal aortic aneurysms in mice. Circulation 121, 1338–1346 (2010).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Rodrigues, R. M. et al. The proteomic and particle composition of human platelet lysate for cell therapy products. J. Cell. Biochem. 123, 1495–1505 (2022).
Nording, H. et al. The C5a/C5a receptor 1 axis controls tissue neovascularization through CXCL4 release from platelets. Nat. Commun. 12, 3352 (2021).
McVey, M. J. et al. Platelet extracellular vesicles mediate transfusion-related acute lung injury by imbalancing the sphingolipid rheostat. Blood 137, 690–701 (2021).
Anselmo, A. et al. Myocardial hypoxic stress mediates functional cardiac extracellular vesicle release. Eur. Heart J. 42, 2780–2792 (2021).
Leonetti, D. et al. Secretion of acid sphingomyelinase and ceramide by endothelial cells contributes to radiation-induced intestinal toxicity. Cancer Res. 80, 2651–2662 (2020).
Zhao, Y. et al. Mesenchymal stem cells regulate inflammatory milieu within degenerative nucleus pulposus cells via p38 MAPK pathway. Exp. Ther. Med. 20, 22 (2020).
Jung, Y. S. et al. Cadmium induces apoptotic cell death through p38 MAPK in brain microvessel endothelial cells. Eur. J. Pharmacol. 578, 11–18 (2008).
Alhasan, A. A. et al. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood 127, e1–e11 (2016).
Elagib, K. E. et al. Neonatal expression of RNA-binding protein IGF2BP3 regulates the human fetal-adult megakaryocyte transition. J. Clin. Invest. 127, 2365–2377 (2017).
Xu, J. et al. Platelet-mimicking nanosponges for functional reversal of antiplatelet agents. Circ. Res. 132, 339–354 (2023).
Lin, C. L. et al. Alkaline ceramidase 1 protects mice from premature hair loss by maintaining the homeostasis of hair follicle stem cells. Stem Cell Rep. 9, 1488–1500 (2017).
Kurtz, T. W., Griffin, K. A., Bidani, A. K., Davisson, R. L. & Hall, J. E. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arterioscler. Thromb. Vasc. Biol. 25, e22–e33 (2005).
Sawada, H., Daugherty, A. & Lu, H. S. Expression of a PCSK9 gain-of-function mutation in C57BL/6J mice to facilitate angiotensin II-induced AAAs. Biomolecules 12, 915 (2022).
Simoes, M. et al. Measurement of neutral ceramidase activity in vitro and in vivo. Anal. Biochem. 643, 114577 (2022).
Liang, Y. et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood 126, 905–914 (2015).
Chen, C. Y., Huang, J. W., Tzu-Chi Lee, C., Lai, W. T. & Huang, Y. B. Long-term outcome of patients with aortic aneurysms taking low-dose aspirin: a population-based cohort study. J. Investig. Med. 61, 1004–1012 (2013).
Acknowledgements
This work was supported by funding from the National Key R&D Program of China (2019YFA0801600, to W.K.) and the National Natural Science Foundation of China (8192100, to W.K.; 82170499, to Y.F.; 82230010, to W.K.; 31930056, to W.K.; and 31925021, to C.J.).
Author information
Authors and Affiliations
Contributions
X.Z. performed most experiments, acquired and analyzed the data and wrote the manuscript. T.Z., W.L. and Y.Z. provided the case–control study samples. J.W. and X.L. contributed to the experiments of ceramidase activity measurement. Z.G. performed the FeCl3-induced thrombosis murine model and tail bleeding time measurement and contributed to the experiment of platelet adhesion and spreading. Y.S. contributed to the experiment of flow cytometry, to data analysis and to platelet extraction. Z.C. performed the elastase-induced AAA model and contributed to lipidomic analysis. L.Y. contributed to the measurement of protein stability and ubiquitination. S.Z. assisted with animal preparation. C.L. and W.W. provided data regarding HuR deficiency in platelets in response to reviewer comments. X.W., J.L. and R.Q. instructed the experiments to evaluate platelet functions. C.J., Y.F. and W.K. designed the study, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Reijo Laaksonen, Lars Maegdefessel, Cungui Mao and Bernhard Nieswandt for their contributions to the peer review of this work. Primary Handling Editor: Gerburg Schwarzer, in collaboration with the Nature Cardiovascular Research team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Lipidomic profiling of platelets from AngII-infused mice.
a-c. Principal component analysis of lipidomic data on phospholipids (a) and quantitative measurement of Ceramides, SM, PC, PE, Lyso-PC and Lyso-PE by mass spectrometry (b-c) in circulating platelets from 16-week-old male ApoE-/- mice infused with saline, AngII (1000 ng/kg/min), AngII plus losartan (in drinking water, 60 mg/kg/day) or AngII plus aspirin (in drinking water, 3 mg/kg/day) for 7 days. Each point represents an individual mouse sample (a). n = 12 mice per group, P values were calculated by Krustal-Wallis test followed by Dunn’s test (b-c). d. Platelets were isolated from 16-week-old male ApoE-/- mice infused with saline or AngII (1000 ng/kg/min) for 7 days, followed by further incubation for 30 min. Mass spectrometric measurement of ceramides in conditional media of isolated mouse platelets. n = 3 biologically independent experiments, P values were calculated by the two-sided Mann-Whitney test; ND, not detected. e. Mass spectrometric measurement of plasma ceramides from 16-week-old male ApoE-/- mice infused with saline or AngII (1000 ng/kg/min) for 7 days. n = 12 mice per group, P values were calculated by two-sided Mann-Whitney test. f. Quantitative measurement of ceramide by mass spectrometry in plasma from 16-week-old male ApoE-/- mice infused with saline or AngII (1000 ng/kg/min) for 28 days. n = 7 mice per group, P values were calculated by the two-sided Mann-Whitney test. Data are presented as mean ± SEM.
Extended Data Fig. 2 Acer1 is downregulated in platelets by AngII.
a. Quantitative measurements of SM, PC, PE, Lyso-PC and Lyso-PE by mass spectrometry in Meg-01 cell-produced PLPs treated with AngII (1 μM), AngII plus losartan (1 μM) or AngII plus aspirin (50 μM) for 3 days. n = 3 independent experiments, P values were calculated by the two-way ANOVA followed by the Bonferroni test. b-e. qRT-PCR evaluation of enzyme genes of ceramide metabolism pathways, including the de novo pathway (b), sphingomyelinase pathway (c), salvage pathway (d) and hydrolysis pathway (e), in PLPs treated with vehicle, AngII (1 μM), AngII and losartan (1 μM), or AngII and aspirin (50 μM) for 24 hours. n = 3 biologically independent experiments, P values were calculated by Krustal-Wallis test followed by Dunn’s test. f. Quantitative measurement of ceramides by mass spectrometry in Meg-01 cell-produced platelet-like particles (PLPs) treated with AngII (1 μM) for different time periods. n = 3 biologically independent experiments, P values were calculated by one-way ANOVA followed by the Bonferroni test. Data are presented as mean ± SEM.
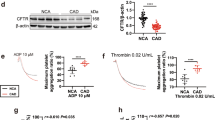
Extended Data Fig. 3 Acer1 is downregulated in Platelets from AAA mice and patients.
a-b. Quantitative measurement of Sphingosine (Sph) by mass spectrometry (a) and S1P by ELISA (b) in platelets isolated from mice infused with saline or AngII (1000 ng/kg/min) for 7 days. n = 5 mice per group. c. Flow cytometric analysis of Acer1 expression in megakarocytes from C57BL6 mice infused with saline or AngII. n = 5 mice per group. d. Western blot analysis of the half-life (t1/2) of Acer1 protein in mouse platelets pretreated with cycloheximide (CHX, 10 μg/mL) for 30 min to inhibit translation, followed with saline (as vehicle) or AngII (1 μM) treatment for different time periods. The relative level of Acer1 expression at the 0 h time point was set as 100%. n = 3 biologically independent experiments. e-f. qRT-PCR (e) and Western blot analysis (f) of Acer1 expression in mouse platelets stimulated with AngII (1 μM) or saline as vehicle for 6 hours in vitro. n = 4 biologically independent samples. g. Alkaline ceramidase activity of mouse platelets stimulated with AngII (1 μM) for 6 hours in vitro. n = 8 biologically independent samples. h. Left: Western blot analysis of Meg-01 cells-produced PLPs transfected with pcDNA3.1 and pcDNA3.1-Acer1 plasmids. Right: Alkaline ceramidase activity of PLPs with or without Acer1 overexpresion. n = 4 biologically independent experiments. i. Alkaline ceramidase activity of platelets from Acer1-/- and littermate WT mice infused with saline or AngII (1000 ng/kg/min) for 7 days. n = 5 biologically independent samples. j-m. qRT-PCR evaluation of enzyme genes of ceramide metabolism pathways, including the de novo pathway (j), sphingomyelinase pathway (k), salvage pathway (l) and hydrolysis pathway (m), in platelets from AAA patients and control individuals (non-AAA). n = 5 biologically independent samples. n. Quantitative measurement of ceramides by mass spectrometry in circulating platelets from Acer1-/- and littermate C57BL6 mice infused with AngII (1000 ng/kg/min) for 7 days. n = 5 mice per group. Data are presented as mean ± SEM. P values were calculated by the two-sided Mann-Whitney test (a-c, e-h, j-n), the two-sided unpaired Student’s t test (d) or ANOVA followed by the Bonferroni test (i).
Extended Data Fig. 4 Acer1 Deficiency in platelets does not affect thoracic aortic aneurysm.
a. Representative morphology of thoracic aortas from AngII-induced Acer1fl/fl PF4-Cretg (n = 10) or Acer1fl/fl (n = 11) mice transduced with AAV-mPCSK9 D377Y and fed with HFHC diet. b. Quantification of maximal aortic diameters, P values were calculated by the two-sided Mann-Whitney test, ns, no significance. Data are presented as mean ± SEM.
Extended Data Fig. 5 Platelet-specific Acer1 deficiency aggravates elastase-induced AAA formation.
a. Representative morphology of infrarenal aortas from elastase-induced Acer1fl/fl PF4-Cretg or littermate Acer1fl/fl mice. n = 7 mice per group. b. The maximal diameters of infrarenal aortas. P value was calculated by the two-sided Mann-Whitney test. c-d. Representative Verhoeff-Van Gieson staining (c) and quantitation of elastin degradation (d) in the aneurysmal aortic walls. P value was calculated by the two-sided Mann-Whitney test. Data are presented as mean ± SEM.
Extended Data Fig. 6 Acer1 overexpression in bone marrow ameliorates AngII-induced AAA formation in mice.
a. Flow cytometry analysis of GFP expression in mouse BMCs with or without 2-day infection of AAV9-GFP (1×108 vg/mL). b-c. Representative Western blot and quantification of Acer1 protein in BMDMs (b) and PBMCs (c) cells treated with vehicle or AngII (1 μM) for 48 hours. n = 3 biologically independent samples, P values were calculated by the two-sided paired Student’s t test. d. Western blot analysis of Acer1 protein in platelets from ApoE-/- mice with bone marrow reconstitution for 28 days by transplantation with AAV9-GFP- or AAV9-Acer1-infected ApoE-/- BMCs. e. Experimental workflow of AAV9 infection in isolated bone marrow cells (BMCs) and bone marrow transplantation in male ApoE-/- mice using infected cells followed by 28-day infusion of AngII (1000 ng/kg/min). n = 10 mice per group. f. Representative morphology of aortas from 16-week-old ApoE-/- male mice transplanted with AAV9-GFP- or AAV9-Acer1-infected BMCs followed by AngII or saline infusion. g. The incidence of AAA. P values were calculated by the two-sided Chi-square test. h. The maximal diameters of abdominal aortas. P values were calculated by two-way ANOVA followed by the Bonferroni test. i-j. Representative Verhoeff-Van Gieson staining (i) and quantitation of elastin degradation (j) in aneurysmal aortic walls. P values were calculated by two-way ANOVA followed by the Bonferroni test. Data are presented as mean ± SEM.
Extended Data Fig. 7 AngII Infusion promotes platelet secretion in mice.
a-d. Washed platelets were isolated from 16-week-old male ApoE-/- mice infused with saline or AngII (1000 ng/kg/min) for 7 days. (a-b) Representative images and quantitative data for platelet adhesion on collagen I (a) and spreading on fibrinogen (b) following thrombin (0.005 U/mL) stimulation for 10, 30 or 60 min. n = 8 mice per group. (c-d) Representative tracings and quantitative data for platelet aggregation induced by thrombin (0.005, 0.006, 0.008 and 0.01 U/mL, C) or collagen I (0.5, 1, 1.5 and 2 μg/mL, D). n = 6 biologically independent samples. e. Washed platelets were isolated from ApoE-/- mice infused with saline, AngII (1000 ng/kg/min), or AngII and aspirin (in drinking water, 2-3 mg/kg/day) for 28 days. Protein microarray analysis of cytokine profiling in supernatants of platelets cultured for 30 min. n = 7 mice per group. f-j. The washed platelets were isolated from ApoE-/- mice infused with saline or AngII for 7 days. (f-g) Representative and quantitative data for thrombin (0.1 U/mL, f) or collagen (2 μg/mL, g)-induced ATP secretion from platelets. n = 3 mice per group. (h) Flow cytometric analysis of cell surface P-selectin (CD62P) using FITC-conjugated antibody and integrin αIIbβ3 activation using Alexa Flour 647-conjugated fibrinogen of platelets from C57BL/6 mice with saline or AngII infusion for 7 days, followed by administeration with thrombin (0.001 U/mL) or saline (as vehicle) for 30 minutes. n = 4 mice per group. (i) Representative flow cytometry of platelet-leukocyte aggregates (PLAs, the percentages of CD45+CD41+ subpopulation out of CD45+ blood cells) and activated β2 integrins (LFA-1 and Mac-1) in PLAs using ICAM-1-Fc and PE-conjugated anti-human IgG1 Fc antibody. (j) Quantitation of PLAs in blood and activated β2 integrins (LFA-1 and Mac-1) in PLAs. n = 5 mice per group. k. Quantitative flow cytometry of P-selectin (CD62P) cell surface translocation of Meg-01 cell-produced platelet-like particles (PLPs) treated with AngII (1 μM) for different time periods. n = 4 biologically independent experiments. l. ELISA of IL-1β and MCP-1 in conditioned media produced by PLPs treated with AngII (1 μM) for different time periods. n = 3 biologically independent experiments. m-n. PLPs were produced followed by stimulation with or without AngII (1 μM), with or without ceranib-2 (10 μM) for 3 days. (m) Flow cytometric analysis of P-selectin on the surface of PLPs. n = 4 biologically independent experiments. (n) THP-1 monocytes were incubated with conditioned media of AngII-stimulated PLPs for 24 hours, and then IL-1β was evaluated by qRT-PCR in THP-1 cells. n = 3 biologically independent experiments. Data are presented as mean ± SEM. P values were calculated by ANOVA followed by the Bonferroni test (a-b, e, h, k-n), two-sided unpaired Student’s t test (f-g) or the two-sided Mann-Whitney test (c-d, j).
Extended Data Fig. 8 Acer1 inhibits platelet secretion during AAA pathogenesis.
a-b. (a) Tail bleeding time of WT and Acer1-/- mice. n = 6 mice per group. (b) Representative Doppler echocardiogram of the blood flow rate in right carotid arteries from WT and Acer1-/- mice following FeCl3 injury. The time at which the flow rate was reduced to 0 mL/min was quantified as the complete occlusion time. n = 8 mice per group. c-h. Washed platelets were isolated from WT and Acer1-/- mice. (c-d) Representative images and quantitative data for platelet adhesion on collagen I (d) and spreading on fibrinogen (d) following thrombin stimulation (0.005 U/mL) for 10, 30 or 60 min. n = 5 biologically independent samples. (e-f) Representative tracings and quantitative data for platelet aggregation induced by thrombin (0.005, 0.006, 0.008 and 0.01 U/mL, e) or collagen I (0.5, 1, 1.5 and 2 μg/mL, f). n = 5 biologically independent samples. (g-h) Representative and quantitative data of thrombin (0.1 U/mL, g) or collagen (2 μg/mL, h)-induced ATP secretion by platelets. n = 3 biologically independent samples. i-j. WT and Acer1-/- mice were infused with AngII (1000 ng/kg/min) for 7 days. (i) Tail bleeding time, n = 6 mice per group. (j) Doppler echocardiogram of the blood flow rate in right carotid arteries following FeCl3 injury. The time at which the flow rate was reduced to 0 mL/min was quantified as the complete occlusion time. n = 8 mice per group. k. Quantitative flow cytometry of P-selectin (CD62P) cell surface translocation of WT or Acer1-/- platelets infused with saline or AngII (1000 ng/kg/min) for 7 days. n = 5 biologically independent samples. l-m. Eight-week-old male Acer1fl/fl PF4-Cretg and their littermates Acer1fl/fl mice were intravenously injected with AAV-mPCSK9 D377Y and switched to a HFHC diet. Two weeks after infection, the mice were infused with AngII (1000 ng/kg/min) for 14 days. n = 3 mice per group. (l) Representative morphology and the maximal diameters of abdominal aortas. (m) Verhoeff-Van Gieson staining on the cross-sections of expanded aortas. Data are presented as mean ± SEM. P values were calculated by the two-sided Mann-Whitney test (a-b, e-f, i-j), the two-sided unpaired Student’s t test (g-h) or ANOVA followed by the Bonferroni test (c-d, k-l).
Extended Data Fig. 9 Ceramides facilitate platelet secretion via p38 MAPK signal.
a-b. Representative Western blot and quantification of phosphorylation of ERK5 (a) and JNK1 (b) in platelets from AAA patients and control individuals (non-AAA). n = 4 biologically independent samples. c-d. Representative Western blot and quantification of p38 MAPK phosphorylation in mice platelets stimulated with C16:0 ceramide at increasing doses (5, 10, 20 μM) for 30 min (c) or with C16:0 ceramide (10 μM) for different time periods (d). n = 4 biologically independent experiments. e. Flow cytometry of cell surface P-selectin (CD62P) in platetes stimulated with C16:0 ceramide (5, 10, 20 μM) for 30 min. n = 3 biologically independent experiments. f. Flow cytometry of cell surface P-selectin (CD62P) in platetes stimulated with DMSO, C16:0 ceramide (10 μM) or C16:0 plus SB202910 (100 nM) for 30 min. n = 4 biologically independent experiments. Data are presented as ± SEM. P values were calculated by the two-sided Mann-Whitney test (a-b) or ANOVA followed by the Bonferroni test (c-f).
Extended Data Fig. 10 AngII downregulates HuR dependently on ubiquitin-mediated proteasomal degradation.
a. qRT-PCR analysis of HuR mRNA in mouse platelets treated with AngII (1 μM) for 6 hours in vitro. n = 4 biologically independent experiments. b. Western blot analysis of the half-life (t1/2) of HuR protein in mouse platelets pretreated with cycloheximide (CHX, 10 μg/mL) for 30 min to inhibit translation, followed with saline (as vehicle) or AngII (1 μM) treatment for different time periods. The relative level of HuR expression at the 0 h time point was set as 100%. n = 3 biologically independent experiments. c. Representative Western blot analysis and quantification of HuR in mouse platelets treated with saline or AngII (1 μM) in the absence or presence of MG132 (10 μM) for 3 hours. n = 4 independent experiments. d. Western blot analysis of HuR ubiquitination in mouse platelets treated with saline (as vehicle), AngII (1 μM), AngII plus losartan (1 μM), or AngII plus aspirin (50 μM) in the presence of MG132 (10 μM) for 3 hours. n = 3 biologically independent experiments. e-f. Representative Western blot analysis and quantification of HuR expression from AT1a-/- and WT platelets treated with saline or AngII (1 μM) (e) as well as WT platelets with U46619 (7 μM) or U46619 plus Terutroban (10 ng/mL) treaments (f) for 3 hours. n = 4 biologically independent experiments. Data are presented as mean ± SEM. P values were calculated by the two-sided Mann-Whitney test (a), the two-sided unpaired Student’s t test (b) or multiple comparisons adjusted ANOVA followed by the Bonferroni test (c, e-f).
Supplementary information
Supplementary Data.
Raw data of mass spectrometry in lipidomics
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 2
Unprocessed western blots and/or gels
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 3
Unprocessed western blots and/or gels
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 4
Unprocessed western blots and/or gels
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 5
Unprocessed western blots and/or gels
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 6
Unprocessed western blots and/or gels
Source Data Extended Data Fig./Table 1
Statistical Source Data
Source Data Extended Data Fig./Table 2
Statistical Source Data
Source Data Extended Data Fig./Table 3
Statistical Source Data
Source Data Extended Data Fig./Table 3
Unprocessed western blots and/or gels
Source Data Extended Data Fig./Table 4
Statistical Source Data
Source Data Extended Data Fig./Table 5
Statistical Source Data
Source Data Extended Data Fig./Table 6
Statistical Source Data
Source Data Extended Data Fig./Table 6
Unprocessed western blots and/or gels
Source Data Extended Data Fig./Table 7
Statistical Source Data
Source Data Extended Data Fig./Table 8
Statistical Source Data
Source Data Extended Data Fig./Table 9
Statistical Source Data
Source Data Extended Data Fig./Table 9
Unprocessed western blots and/or gels
Source Data Extended Data Fig./Table 10
Statistical Source Data
Source Data Extended Data Fig./Table 10
Unprocessed western blots and/or gels
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Gong, Z., Shen, Y. et al. Alkaline ceramidase 1–mediated platelet ceramide catabolism mitigates vascular inflammation and abdominal aortic aneurysm formation. Nat Cardiovasc Res 2, 1173–1189 (2023). https://doi.org/10.1038/s44161-023-00364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00364-1
This article is cited by
-
Platelet ceramides drive thrombo-inflammation in aortic aneurysm formation
Nature Cardiovascular Research (2023)