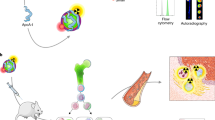
Abstract
Among the diverse populations of myeloid cells that reside within the healthy and diseased heart, C-C chemokine receptor type 2 (CCR2) is specifically expressed on inflammatory populations of monocytes and macrophages that contribute to the development and progression of heart failure1,2,3,4. Here, we evaluated a peptide-based imaging probe (64Cu-DOTA-ECL1i) that specifically recognizes CCR2+ monocytes and macrophages for human cardiac imaging. Compared to healthy controls, 64Cu-DOTA-ECL1i heart uptake was increased in individuals after acute myocardial infarction, predominately localized within the infarct area, and was associated with impaired myocardial wall motion. These findings establish the feasibility of molecular imaging of CCR2 expression to visualize inflammatory monocytes and macrophages in the injured human heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All source data are provided in the supplementary materials; additional details are available from the authors upon reasonable request.
Code availability
No custom code was used in this manuscript.
References
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014).
Epelman, S., Lavine, K. J. & Randolph, G. J. Origin and functions of tissue macrophages. Immunity 41, 21–35 (2014).
Bajpai, G. et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 24, 1234–1245 (2018).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Mann, D. L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ. Res. 116, 1254–1268 (2015).
van der Laan, A. M. et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am. Heart J. 163, 57–65 (2012).
Mariani, M. et al. Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. Eur. Heart J. 27, 2511–2515 (2006).
Rischpler, C. et al. Prospective evaluation of 18F-fluorodeoxyglucose uptake in postischemic myocardium by simultaneous positron emission tomography/magnetic resonance imaging as a prognostic marker of functional outcome. Circ. Cardiovasc. Imaging 9, e004316 (2016).
Wollenweber, T. et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ. Cardiovasc. Imaging 7, 811–818 (2014).
Thackeray, J. T. & Bengel, F. M. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: a determinant of subsequent remodeling or recovery. JACC Cardiovasc. Imaging 11, 1340–1355 (2018).
Kunze, K. P. et al. Quantitative cardiovascular magnetic resonance: extracellular volume, native T1 and 18F-FDG PET/CMR imaging in patients after revascularized myocardial infarction and association with markers of myocardial damage and systemic inflammation. J. Cardiovasc. Magn. Reson. 20, 33 (2018).
Spieker, M. et al. T2 mapping cardiovascular magnetic resonance identifies the presence of myocardial inflammation in patients with dilated cardiomyopathy as compared to endomyocardial biopsy. Eur. Heart J. Cardiovasc. Imaging 19, 574–582 (2018).
Leuschner, F. et al. Silencing of CCR2 in myocarditis. Eur. Heart J. 36, 1478–1488 (2015).
Majmudar, M. D. et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 127, 2038–2046 (2013).
Chung, E. S. et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Parrillo, J. E. et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N. Engl. J. Med. 321, 1061–1068 (1989).
Giugliano, G. R., Giugliano, R. P., Gibson, C. M. & Kuntz, R. E. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am. J. Cardiol. 91, 1055–1059 (2003).
Han, K. H., Tangirala, R. K., Green, S. R. & Quehenberger, O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler. Thromb. Vasc. Biol. 18, 1983–1991 (1998).
Auvynet, C. et al. ECL1i, d(LGTFLKC), a novel, small peptide that specifically inhibits CCL2-dependent migration. FASEB J. 30, 2370–2381 (2016).
Heo, G. S. et al. Molecular imaging visualizes recruitment of inflammatory monocytes and macrophages to the injured heart. Circ. Res. 124, 881–890 (2019).
Heo, G. S. et al. Targeted PET imaging of chemokine receptor 2-positive monocytes and macrophages in the injured heart. J. Nucl. Med. 62, 111–114 (2021).
Li, W. et al. Visualization of monocytic cells in regressing atherosclerotic plaques by intravital 2-photon and positron emission tomography-based imaging—brief report. Arterioscler. Thromb. Vasc. Biol. 38, 1030–1036 (2018).
Hsiao, H.-M. et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J. Clin. Invest. 128, 2833–2847 (2018).
Brody, S. L. et al. Chemokine receptor 2-targeted molecular imaging in pulmonary fibrosis. A clinical trial. Am. J. Respir. Crit. Care Med. 203, 78–89 (2021).
Thackeray, J. T. et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc. Imaging 8, 1417–1426 (2015).
Gibb, A. A., Lazaropoulos, M. P. & Elrod, J. W. Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circ. Res. 127, 427–447 (2020).
Lombardi, A. A. et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat. Commun. 10, 4509 (2019).
Tavakoli, S. et al. Differential regulation of macrophage glucose metabolism by macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: implications for 18F FDG PET imaging of vessel wall inflammation. Radiology 283, 87–97 (2017).
Koenig, A. L. et al. Single-cell transcriptomics reveals cell-type-specific diversification in human heart failure. Nat. Cardiovasc. Res. 1, 263–280 (2022).
Sicklinger, F. et al. Basophils balance healing after myocardial infarction via IL-4/IL-13. J. Clin. Invest. 131, e136778 (2021).
Sager, H. B. et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ. Res. 119, 853–864 (2016).
Dorbala, S. et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J. Nucl. Cardiol. 25, 1784–1846 (2018).
Acknowledgements
K.J.L. is supported by grants from the National Institutes of Health (NIH) (nos. HL161185, HL150891 and HL151078), the Children’s Discovery Institute (no. PM-LI-2019-829), Burroughs Welcome Fund (no. 1014782), Leducq Foundation (no. 20CVD02) and generous gifts through Washington University and Barnes-Jewish Hospital. S.L.B. is supported for these studies by the NIH (no. HL151685) and the Barnes-Jewish Hospital Foundation. Y.L. is supported by grants from the NIH (nos. HL145212, HL150891, HL153436, HL151685 and EB025815).
Author information
Authors and Affiliations
Contributions
K.J.L., Y.L. and R.J.G. conceived and designed the study and wrote the paper. Y.L., D.S., H.L., L.D., Xiaohui Zhang., G.S.H. and Xiuli Zhang were responsible for the radiotracer synthesis. M.H., K.H. and R.J.G. were responsible for regulatory compliance and patient enrollment. R.L., P.K.W. and R.J.G. were responsible for image analysis and interpretation. C.C. provided the ECL1i peptide. S.L.B., D.K. and R.J.G. assisted with writing the paper and interpreting the data.
Corresponding authors
Ethics declarations
Competing interests
K.J.L. serves as a consultant for Implicit Bioscience and Medtronic and is the recipient of sponsored research agreements with Amgen and Novartis. K.J.L., D.K., S.L.B., R.J.G. and Y.L. are inventors on a pending patent entitled ‘Methods for detecting CCR2 receptors’ (application no. US17/001,857). This patent was submitted by Washington University School of Medicine and pertains to the CCR2 imaging tracer used in the manuscript. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks R.H.J.A. Slart, C. Schulz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: E. Martini, in collaboration with the Nature Cardiovascular Research team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro cell binding assays of 64Cu-DOTA-ECL1i in THP-1 cells and 293 T cells.
Data are representative of at least three independent experiments from THP cells (left, express CCR2) and HEK293T cells (right, do not express CCR2). Cells (2 ×106) were incubated with approximately 37 KBq of 64Cu-DOTA-ECL1i with the indicated concentrations of cold DOTA-ECL1i at room temperature for 1 h. n = 3 technical replicates. Error bars are standard deviation.
Extended Data Fig. 2 In vitro cell binding assays of 64Cu-DOTA-ECL1i in human immune cells.
Data are representative of at least three independent experiments from 2 donors. *** p < 0.001, **** p < 0.0001. 1-way ANOVA. CPM: counts per minute. n = 3 technical replicates. Error bars are standard deviation.
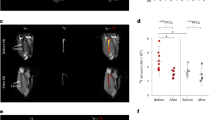
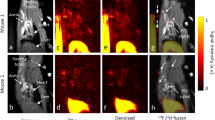
Extended Data Fig. 3 CCR2 imaging in the other control subjects.
99mTc-Tetrofosmin (99mTc) SPECT/CT and differential 64Cu-DOTA-ECL1i (CCR2) PET/CT fused images as described in Fig. 1. SPECT/CT perfusion and CCR2 PET/CT images are co-registered and comparative anatomic slices displayed. Differential 64Cu-DOTA-ECL1i images are corrected for blood activity. Color scale indicates normalized relative tracer uptake.
Extended Data Fig. 4 CCR2 imaging in the STEMI patients.
99mTc-Tetrofosmin (99mTc) SPECT/CT and differential 64Cu-DOTA-ECL1i (CCR2) PET/CT fused images as described in Fig. 1. SPECT/CT perfusion and CCR2 PET/CT images are co-registered and comparative anatomic slices displayed. Differential 64Cu-DOTA-ECL1i images are corrected for blood activity. Green and red arrows denote the infarct region. Color scale indicates normalized relative tracer uptake.
Supplementary information
Supplementary Information
Clinical protocol.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lavine, K.J., Sultan, D., Luehmann, H. et al. CCR2 imaging in human ST-segment elevation myocardial infarction. Nat Cardiovasc Res 2, 874–880 (2023). https://doi.org/10.1038/s44161-023-00335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00335-6