Abstract
Cardiac hypertrophy leads to myocardial dysfunction and represents a serious threat to global public health security. Deubiquitinating enzymes (DUBs) mainly maintain the stability of substrate proteins and are essential to cardiac pathophysiology. Here, we explored the role and regulating mechanism of a DUB, Josephin domain-containing protein 2 (JOSD2), in cardiac hypertrophy. We found that JOSD2 expression was significantly upregulated in hypertrophic myocardium. Josd2 gene knockout aggravated cardiac dysfunction and hypertrophy in mice, whereas cardiac overexpression of JOSD2 mediated by the AAV9 vector prevented angiotensin II-induced cardiac hypertrophy. A comprehensive proteome-wide quantitative analysis identified sarco/endoplasmic reticulum calcium ATPase 2a (SERCA2a) as a key substrate of JOSD2. Mechanistically, JOSD2 mediates SERCA2a deubiquitination, enhancing the stability of SERCA2a. By regulating SERCA2a, JOSD2 deficiency impairs calcium handling and promotes hypertrophy in primary cardiomyocytes. Our findings highlight the promise of JOSD2 as a beneficial therapeutic target for hypertrophic cardiomyopathy and provide an additional strategy for SERCA2a-targeted therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are included in the main article and associated files. Source data are provided with this paper. The proteomics raw data have been deposited to the ProteomeXchange Consortium via the PRIDE repository (identifier PXD043382).
References
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
McKinsey, T. A. & Kass, D. A. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat. Rev. Drug Discov. 6, 617–635 (2007).
Yan, K., Wang, K. & Li, P. The role of post-translational modifications in cardiac hypertrophy. J. Cell. Mol. Med. 23, 3795–3807 (2019).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Mevissen, T. E. T. & Komander, D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 (2017).
Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev. Cardiol. 17, 341–359 (2020).
Zeng, C. et al. Machado–Joseph deubiquitinases: from cellular functions to potential therapy targets. Front. Pharmacol. 11, 1311 (2020).
Hutchins, A. P., Liu, S., Diez, D. & Miranda-Saavedra, D. The repertoires of ubiquitinating and deubiquitinating enzymes in eukaryotic genomes. Mol. Biol. Evol. 30, 1172–1187 (2013).
Haberhausen, G., Damian, M. S., Leweke, F. & Müller, U. Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado–Joseph disease (MJD). J. Neurol. Sci. 132, 71–75 (1995).
Zhang, B. et al. PHGDH defines a metabolic subtype in lung adenocarcinomas with poor prognosis. Cell Rep. 19, 2289–2303 (2017).
Krassikova, L. et al. The deubiquitinase JOSD2 is a positive regulator of glucose metabolism. Cell Death Differ. 28, 1091–1109 (2021).
Qian, M. et al. Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression. Acta Pharm. Sin. B 11, 4008–4019 (2021).
Huang, Y. et al. Deubiquitinating enzyme JOSD2 promotes hepatocellular carcinoma progression through interacting with and inhibiting CTNNB1 degradation. Cell Biol. Int. https://doi.org/10.1002/cbin.11812 (2022).
Lei, H. et al. JOSD2 regulates PKM2 nuclear translocation and reduces acute myeloid leukemia progression. Exp. Hematol. Oncol. 11, 42 (2022).
Grasty, K. C., Weeks, S. D. & Loll, P. J. Structural insights into the activity and regulation of human Josephin-2. J. Struct. Biol. X 3, 100011 (2019).
Kranias, E. G. & Hajjar, R. J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660 (2012).
Zhihao, L. et al. SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail. Rev. 25, 523–535 (2020).
Tilemann, L., Ishikawa, K., Weber, T. & Hajjar, R. J. Gene therapy for heart failure. Circ. Res. 110, 777–793 (2012).
Sitsel, A. et al. Structures of the heart specific SERCA2a Ca2+-ATPase. EMBO J. https://doi.org/10.15252/embj.2018100020 (2019).
Fan, Y. et al. Phosphoproteomic analysis of neonatal regenerative myocardium revealed important roles of checkpoint kinase 1 via activating mammalian target of rapamycin C1/ribosomal protein S6 kinase b-1 pathway. Circulation 141, 1554–1569 (2020).
He, B. et al. Ubiquitin-specific protease 4 is an endogenous negative regulator of pathological cardiac hypertrophy. Hypertension 67, 1237–1248 (2016).
Ying, X. et al. Novel protective role for ubiquitin-specific protease 18 in pathological cardiac remodeling. Hypertension 68, 1160–1170 (2016).
Huang, H. et al. Tumor suppressor A20 protects against cardiac hypertrophy and fibrosis by blocking transforming growth factor-b-activated kinase 1-dependent signaling. Hypertension 56, 232–239 (2010).
Kho, C. et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 (2011).
Ye, J. et al. Programmed cell death 5 provides negative feedback on cardiac hypertrophy through the stabilization of sarco/endoplasmic reticulum Ca2+-ATPase 2a protein. Hypertension 72, 889–901 (2018).
Cai, B. et al. Long noncoding RNA–DACH1 (dachshund homolog 1) regulates cardiac function by inhibiting SERCA2a (sarcoplasmic reticulum calcium ATPase 2a). Hypertension 74, 833–842 (2019).
Zsebo, K. et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ. Res. 114, 101–108 (2014).
Greenberg, B. et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387, 1178–1186 (2016).
Zhou, L. et al. Excessive deubiquitination of NLRP3-R779C variant contributes to very-early-onset inflammatory bowel disease development. J. Allergy Clin. Immunol. 147, 267–279 (2021).
Han, J. et al. GSDMD (gasdermin D) mediates pathological cardiac hypertrophy and generates a feed-forward amplification cascade via mitochondria–STING (stimulator of interferon genes) axis. Hypertension 79, 2505–2518 (2022).
Ye, B. et al. Gasdermin D mediates doxorubicin-induced cardiomyocyte pyroptosis and cardiotoxicity via directly binding to doxorubicin and changes in mitochondrial damage. Transl. Res. 248, 36–50 (2022).
Ackers-Johnson, M. et al. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ. Res. 119, 909–920 (2016).
Acknowledgements
We thank L. Hou and Y. Dong from the Scientific Research Center of Wenzhou Medical University for their help in echocardiography. This study was supported by grants from the National Natural Science Foundation of China (81930108 to G.L., 81970338 to Y.W., 82271347 to G.W. and 82170373 to Y.W.).
Author information
Authors and Affiliations
Contributions
G.L., Y.W. and J.H. contributed to the literature search and study design. J.H., G.W. and G.L. participated in the drafting of the article. J.H., Z.F., B.H., B.Y., W.L., Y.J. and X.H. performed the experiments. B.H., X.W. and Y.W. revised the manuscript. J.H. and Z.F. contributed to data collection and analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of JOSD2 as a regulator in cardiac hypertrophy.
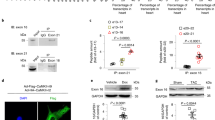
(a) The expression profile of Machado-joseph deubiquitinases (MJDs) family in heart tissues of Ang II mice. Construction of transcriptome sequencing library and transcriptome sequencing was completed by LC-Bio Technology Co., Ltd. (Hangzhou, China). mRNA sequences number was normalized by fragments per kilobase million (FPKM) (n = 3). (b) The silence efficiency of Josd1, Josd2 and Ataxin-3 in NRPCs was verified by RT-qPCR (NC=negative control; n = 3). For a and b, P values were determined by two-tailed unpaired t-test and data are presented as mean ± SEM. (c) Representative immunoblot for JOSD2 in Ang II-induced cardiac fibroblasts (n = 3). (d) Representative immunofluorescence staining for JOSD2 (green) and α-actin (red), vimentin (red) or CD68 (red) in heart sections from Ang II infusion or TAC operation mice. Merged images (yellow) showing colocalization (n = 3).
Extended Data Fig. 2 JOSD2 deficiency promotes TAC-induced cardiac hypertrophy and dysfunction.
Josd2−/− mice and WT mice were undergoing TAC or sham operations for 4 weeks. (a) Representative flow doppler ultrasound images of the aortic arch (left) and transverse aortic flow velocity (right) in Sham or TAC mice. (b) Mice body weight during study. (c) Representative images of HE staining of heart sections (longitudinal). (d,e) Representative images of Masson trichrome staining of the hearts sections and quantitative analysis. (f,g) RT-qPCR analysis of Col-1a1, Tgf-β, Myh7, Bnp and Anp, in cardiac tissues. (n = 6 for each group. For a, P value was determined by two-tailed unpaired t-test. For e, f and g, P values were determined by one-way ANOVA with Bonferroni’s correction. For a,b, e, f and g, data are presented as mean ± SEM).
Extended Data Fig. 3 JOSD2 knockout aggravates myocardial dysfunction and hypertrophy induced by Ang II.
Josd2−/− mice and WT mice were subcutaneously infused by osmotic mini-pumps with Ang II (1000 ng/kg/min) or normal saline (sham) for 4 weeks. (a, b) Ang II levels were detected in heart tissue and serum by ELISA kit (E-EL-H0326c, Elabscience Biotechnology) (n = 6 for each group). (c, d) Mice systolic blood pressure (SBP) and body weight during study (n = 3-6). (e) Representative images of HE staining of heart sections (longitudinal). (f, g) Representative images of Masson trichrome staining of the hearts sections and quantitative analysis (n = 6 for each group). (h) RT-qPCR analysis of Myh7, Anp, Bnp, Col-1a1 and Tgf-β in cardiac tissues (n = 6 for each group). (For a,b,c, g and h, P values were determined by one-way ANOVA with Bonferroni’s correction. For a,b,c,d, g and h, data are presented as mean ± SEM).
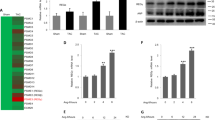
Extended Data Fig. 4 Identification of SERCA2a as a potential substrate of JOSD2.
(a) The expression efficiency of Myc- SERCA2a in NIH/3T3 was verified by immunoblot. (EV= empty vector) (b) Co-IP of JOSD2 and SERCA2a in heart tissues treated with TAC (n = 3). (c) Representative immunoblot of SERCA2a in heart tissue and quantitative analysis (n = 3/groups, P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± SEM).
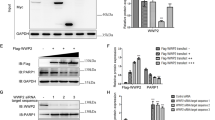
Extended Data Fig. 5 JOSD2 regulates the ubiquitination of SERCA2a.
(a) Myc-SERCA2a and HA-Ub were transfected into cardiomyocytes together with Flag-JOSD2 and then subjected to 10 μM MG132 for 2 h. Lysates were subjected to Co-IP with anti-Myc, which was followed by immunoblot with an anti-HA (n = 3). (b) Myc-SERCA2a and HA-Ub were transfected into cardiomyocytes together with siJOSD2 and then subjected to 10 μM MG132 for 2 h. Lysates were subjected to Co-IP with anti-Myc, which was followed by immunoblot with an anti-HA (n = 3). (c) Lysates of heart tissue from Josd2−/− mice were subjected to Co-IP with anti-SERCA2a, which was followed by immunoblot with an anti-Ub (n = 3). (d) Myc-SERCA2a and HA-K63 were transfected into NIH/3T3 together with Flag-JOSD2 and then subjected to 10 μM MG132 for 2 h. Lysates were subjected to Co-IP with anti-Myc, which was followed by immunoblot with an anti-HA (n = 3). (e) General E3 enzymes ubiquitination prediction for SERCA2a (GPSUber: http://gpsuber.biocuckoo.cn/index.php).
Extended Data Fig. 6 JOSD2 regulates calcium (Ca2+) handling in cardiomyocytes.
(a) Specimen records of caffeine (10 mM)- induced determination of sarcoplasmic reticulum (SR) Ca2+ content in AVCMs isolated from WT or Josd2−/− mice under TAC treatment. (b) The expression efficiency of JOSD2 in NRPCs transfecting with plasmids (left) or siRNA (right) was verified by immunoblot. (n = 3, EV= empty vector, NC=negative control) (c) Representative confocal images and recordings of cytosolic Ca2+ as determined by the Fluo-4 AM fluorescence signal in NRPCs during spontaneous beats. NRPCs were transfected with plasmids (Upper) or siRNA (Below) before Ang II (1 μM) stimulation for 12 h (n = 6, P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± SEM).
Extended Data Fig. 7 Cardiac-specific overexpression of JOSD2 or SERCA2a in Ang II-induced mice.
AAV9 over-expressing JOSD2 (JOSD2OE), SERCA2a (SERCA2aOE), or vehicle (EV) were injected in WT or Josd2−/− mice through tail vein (a total dose of 2E + 11 vg, injected twice, 4 weeks before osmotic pumps implantation). These mice were then implanted with Ang II osmotic pumps. (a, b) Representative immunoblot of Flag-JOSD2 and SERCA2a in heart tissues. (c) Lysates of heart tissue from JOSD2OE mice were subjected to Co-IP with anti-SERCA2a, which was followed by immunoblot with an anti-Ub. (d, e) Mice body weight (n = 6 for each group) and systolic blood pressure (SBP, n = 3-4) during study. (For d and e, data are presented as mean ± SEM).
Extended Data Fig. 8 JOSD2 prevents cardiac hypertrophy via regulating SERCA2a in vivo.
(a) Representative images of HE staining of heart sections (longitudinal). (b, c) Representative images of Masson trichrome staining of the hearts sections and quantitative analysis. (d, e) RT-qPCR analysis of Myh7, Bnp, Anp, Col-1a1 and Tgf-β in cardiac tissues. (n = 6 for each group. For c, d and e, P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± SEM).
Extended Data Fig. 9 JOSD2OE or SERCA2aOE by AAV9 did not affect cardiac function and hypertrophy at the basal condition.
AAV9 over-expressing JOSD2 (JOSD2OE), SERCA2a (SERCA2aOE), or vehicle (EV) were injected in WT mice through tail vein (a total dose of 2E + 11 vg, 4 weeks before osmotic pumps implantation). These mice were then implanted with normal saline osmotic pumps as sham operation. (a) Representative echocardiographic images of left ventricular M-mode and PW doppler. (b-d) Ejection fraction (EF), fractional shortening (FS) and ratio of E and A peak velocity (E/A). (e) Representative images of whole heart. (f, g) Heart weight to body weight ratio (HW/BW), heart weight to tibial length ratio (HW/TL). (h) Representative images of HE staining of the heart tissues were shown. (i, j) Representative images and quantitative analysis of WAG staining. (k, l) Representative images of Masson trichrome staining of the hearts sections and quantitative analysis. (n = 6 for each group. For b, c, d, f, g, j and l, P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± SEM).
Extended Data Fig. 10 JOSD2OE by AAV9 ameliorated TAC-induced established cardiac dysfunction.
(a) WT mice were undergoing TAC operation for 6 weeks. At TAC 2-week, AAV9 (EV or JOSD2OE, a total dose of 2E + 11 vg, tail vein) were injected. Echocardiography was performed at TAC 0, 4, 6-week. Mice were harvested at TAC 6-week. (b) Ejection fraction (EF), fractional shortening (FS) and ratio of E and A peak velocity (E/A) at TAC 0, 4, 6-week. (c) Representative immunoblot of Flag-JOSD2 in heart tissues since TAC 2-week. (d, e) Representative echocardiographic images of left ventricular M-mode and PW doppler at TAC 0, 4, 6-week. (f) Representative images of whole heart at TAC 6-week. (g) Representative images of HE staining of the heart tissues at TAC 6-week were shown. (h, i) Representative images and quantitative analysis of WAG staining at TAC 6-week. (j, k) Representative images of Masson trichrome staining of the hearts sections and quantitative analysis. (n = 6 for each group. For b, P values were determined by two-way ANOVA with Bonferroni’s correction. For i and k, P values were determined by two-tailed unpaired t-test. For b, i and k, data are presented as mean ± SEM).
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Supplementary Data
Statistical Data for Supplementary Tables 2–5.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Source Data Numeric source data for Figs. 1–7 and Extended Data Figs. 1–10.
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, J., Fang, Z., Han, B. et al. Deubiquitinase JOSD2 improves calcium handling and attenuates cardiac hypertrophy and dysfunction by stabilizing SERCA2a in cardiomyocytes. Nat Cardiovasc Res 2, 764–777 (2023). https://doi.org/10.1038/s44161-023-00313-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00313-y