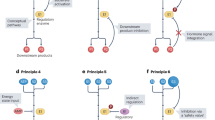
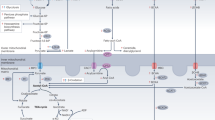
Abstract
The heart is the most metabolically active organ in the body, sustaining a continuous and high flux of nutrient catabolism via oxidative phosphorylation. The nature and relative contribution of these fuels have been studied extensively for decades. By contrast, less attention has been placed on how intermediate metabolites generated from this catabolism affect intracellular signaling. Numerous metabolites, including intermediates of glycolysis and the tricarboxylic acid (TCA) cycle, nucleotides, amino acids, fatty acids and ketones, are increasingly appreciated to affect signaling in the heart, via various mechanisms ranging from protein–metabolite interactions to modifying epigenetic marks. We review here the current state of knowledge of intermediate metabolite signaling in the heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021). Excellent recent review on intermediate metabolism in the heart.
Sullivan, L. B., Gui, D. Y. & Vander Heiden, M. G. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat. Rev. Cancer 16, 680–693 (2016).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963). Classic paper describing the antagonistic relationship between fatty acid and carbohydrate use in the heart.
Hue, L. & Taegtmeyer, H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 297, E578–E591 (2009).
Alrob, O. A. et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc. Res. 103, 485–497 (2014).
Gibala, M. J., MacLean, D. A., Graham, T. E. & Saltin, B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am. J. Physiol. 275, E235–E242 (1998).
Bartman, C. R., TeSlaa, T. & Rabinowitz, J. D. Quantitative flux analysis in mammals. Nat. Metab. 3, 896–908 (2021). Comprehensive and quantitative analyses of whole-body fuel flux in mice.
Vendelin, M. & Birkedal, R. Anisotropic diffusion of fluorescently labeled ATP in rat cardiomyocytes determined by raster image correlation spectroscopy. Am. J. Physiol. Cell Physiol. 295, C1302–C1315 (2008).
Norris, J. L. & Caprioli, R. M. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem. Rev. 113, 2309–2342 (2013).
Gilmore, I. S., Heiles, S. & Pieterse, C. L. Metabolic imaging at the single-cell scale: recent advances in mass spectrometry imaging. Annu. Rev. Anal. Chem. 12, 201–224 (2019).
Sahlin, K., Katz, A. & Broberg, S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 259, C834–C841 (1990).
Flam, E. et al. Integrated landscape of cardiac metabolism in end-stage human non-ischemic dilated cardiomyopathy. Nat. Cardiovasc. Res. 1, 817–829 (2022).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014). Paradigm-shifting demonstration of the key role succinate that plays in cardiac I/R injury.
Martin, J. L. et al. Succinate accumulation drives ischaemia–reperfusion injury during organ transplantation. Nat. Metab. 1, 966–974 (2019).
Milliken, A. S., Nadtochiy, S. M. & Brookes, P. S. Inhibiting succinate release worsens cardiac reperfusion injury by enhancing mitochondrial reactive oxygen species generation. J. Am. Heart Assoc. 11, e026135 (2022).
Murphy, M. P. & Chouchani, E. T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 18, 461–469 (2022).
Jr Kaelin, W. G. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Moslehi, J. et al. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation 122, 1004–1016 (2010).
Knutson, A. K., Williams, A. L., Boisvert, W. A. & Shohet, R. V. HIF in the heart: development, metabolism, ischemia, and atherosclerosis. J. Clin. Invest. 131, e137557 (2021).
Sano, M. et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448 (2007).
Koivunen, P. et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 282, 4524–4532 (2007).
Dodd, M. S. et al. Fatty acids prevent hypoxia-inducible factor-1α signaling through decreased succinate in diabetes. JACC Basic Transl. Sci. 3, 485–498 (2018).
Prag, H. A. et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res. 117, 1188–1201 (2021).
Pepin, M. E. et al. DNA methylation reprograms cardiac metabolic gene expression in end-stage human heart failure. Am. J. Physiol. Heart Circ. Physiol. 317, H674–H684 (2019).
Haas, J. et al. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol. Med. 5, 413–429 (2013).
Xiao, D. et al. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc. Res. 101, 373–382 (2014).
Kaneda, R. et al. Genome-wide histone methylation profile for heart failure. Genes Cells 14, 69–77 (2009).
Rosa-Garrido, M., Chapski, D. J. & Vondriska, T. M. Epigenomes in cardiovascular disease. Circ. Res. 122, 1586–1607 (2018).
Zhang, Q. J. et al. The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J. Clin. Invest. 121, 2447–2456 (2011).
Kranendijk, M., Struys, E. A., Salomons, G. S., Van der Knaap, M. S. & Jakobs, C. Progress in understanding 2-hydroxyglutaric acidurias. J. Inherit. Metab. Dis. 35, 571–587 (2012).
Akbay, E. A. et al. d-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev. 28, 479–490 (2014).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009). Discovery of neomorphic mutations in IDH1 that promote enzymatic production of 2-hydroglutarate, which in turn powerfully inhibits various dioxygenases.
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Karlstaedt, A. et al. Oncometabolite d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc. Natl Acad. Sci. USA 113, 10436–10441 (2016). Established connection between 2-hydroxyglutarate and cardiac dysfunction.
Latini, A. et al. Mitochondrial energy metabolism is markedly impaired by d-2-hydroxyglutaric acid in rat tissues. Mol. Genet. Metab. 86, 188–199 (2005).
Diotallevi, M., Ayaz, F., Nicol, T. & Crabtree, M. J. Itaconate as an inflammatory mediator and therapeutic target in cardiovascular medicine. Biochem. Soc. Trans. 49, 2189–2198 (2021).
Murphy, M. P. & O’Neill, L. A. J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018).
Michelucci, A. et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl Acad. Sci. USA 110, 7820–7825 (2013).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Cordes, T. et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 291, 14274–14284 (2016).
Wu, R. et al. Aconitate decarboxylase 1 is a mediator of polymicrobial sepsis. Sci. Transl. Med. 14, eabo2028 (2022).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 556, 501–504 (2018).
Swain, A. et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat. Metab. 2, 594–602 (2020).
Song, H. et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. EBioMedicine 57, 102832 (2020).
Qin, W. et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol. 15, 983–991 (2019).
Nakkala, J. R. et al. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardic infarction. Small 17, e2006992 (2021).
Shan, Q. et al. Protective effects of dimethyl itaconate in mice acute cardiotoxicity induced by doxorubicin. Biochem. Biophys. Res. Commun. 517, 538–544 (2019).
Ashrafian, H. et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 15, 361–371 (2012).
Ali, H. R. et al. Defining decreased protein succinylation of failing human cardiac myofibrils in ischemic cardiomyopathy. J. Mol. Cell. Cardiol. 138, 304–317 (2020).
Laukka, T. et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem. 291, 4256–4265 (2016).
Taegtmeyer, H., Roberts, A. F. & Raine, A. E. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J. Am. Coll. Cardiol. 6, 864–870 (1985).
Sen, S. et al. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J. Am. Heart Assoc. 2, e004796 (2013).
Shioi, T. et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107, 1664–1670 (2003).
Sharma, S., Guthrie, P. H., Chan, S. S., Haq, S. & Taegtmeyer, H. Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart. Cardiovasc. Res. 76, 71–80 (2007).
Roberts, D. J., Tan-Sah, V. P., Ding, E. Y., Smith, J. M. & Miyamoto, S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell 53, 521–533 (2014).
Miyamoto, S., Murphy, A. N. & Brown, J. H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 15, 521–529 (2008).
Sun, L., Shukair, S., Naik, T. J., Moazed, F. & Ardehali, H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell. Biol. 28, 1007–1017 (2008).
Karlstaedt, A., Khanna, R., Thangam, M. & Taegtmeyer, H. Glucose 6-phosphate accumulates via phosphoglucose isomerase inhibition in heart muscle. Circ. Res. 126, 60–74 (2020).
Olson, A. K., Bouchard, B., Zhu, W. Z., Chatham, J. C. & Des Rosiers, C. First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart. J. Biol. Chem. 295, 2018–2033 (2020).
Lunde, I. G. et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genomics 44, 162–172 (2012).
Prakoso, D. et al. Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc. Res. 118, 212–225 (2021).
Wang, Z. V. et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156, 1179–1192 (2014). Initial demonstration of a link between the endoplasmic reticulum stress response and glycolytic side programs, including the HBP, promoting cardiac hypertrophy.
Tran, D. H. et al. Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat. Commun. 11, 1771 (2020).
Gelinas, R. et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 9, 374 (2018).
Erickson, J. R. et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502, 372–376 (2013).
Kronlage, M. et al. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation 140, 580–594 (2019).
Medford, H. M., Porter, K. & Marsh, S. A. Immediate effects of a single exercise bout on protein O-GlcNAcylation and chromatin regulation of cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 305, H114–H123 (2013).
Hegyi, B. et al. CaMKII serine 280 O-GlcNAcylation links diabetic hyperglycemia to proarrhythmia. Circ. Res. 129, 98–113 (2021).
Lu, S. et al. Hyperglycemia acutely increases cytosolic reactive oxygen species via O-linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ. Res. 126, e80–e96 (2020).
Chatham, J. C., Zhang, J. & Wende, A. R. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol. Rev. 101, 427–493 (2021).
Lauzier, B. et al. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J. Mol. Cell. Cardiol. 55, 92–100 (2013).
Lehmann, L. H. et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat. Med. 24, 62–72 (2018).
Schalkwijk, C. G. & Stehouwer, C. D. A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 100, 407–461 (2020).
Bollong, M. J. et al. A metabolite-derived protein modification integrates glycolysis with KEAP1–NRF2 signalling. Nature 562, 600–604 (2018). Demonstration of how methyl glyoxylate, the most reactive known glycolytic metabolite and leading cause of AGEs, triggers a cellular hormetic response via the KEAP–NRF2 pathway.
Papadaki, M. et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI Insight 3, e121264 (2018).
Tian, C. et al. Reactive carbonyl species and their roles in sarcoplasmic reticulum Ca2+ cycling defect in the diabetic heart. Heart Fail. Rev. 19, 101–112 (2014).
Thangarajah, H. et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc. Natl Acad. Sci. USA 106, 13505–13510 (2009).
Bento, C. F. et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS ONE 5, e15062 (2010).
Ramasamy, R. & Schmidt, A. M. Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Curr. Heart Fail. Rep. 9, 107–116 (2012).
Shimizu, Y. et al. Role of DJ-1 in modulating glycative stress in heart failure. J. Am. Heart Assoc. 9, e014691 (2020).
Qi, D. & Young, L. H. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol. Metab. 26, 422–429 (2015).
Dyck, J. R. & Lopaschuk, G. D. AMPK alterations in cardiac physiology and pathology: enemy or ally? J. Physiol. 574, 95–112 (2006).
Wang, J., Gareri, C. & Rockman, H. A. G-protein-coupled receptors in heart disease. Circ. Res. 123, 716–735 (2018).
Downey, J. M., Davis, A. M. & Cohen, M. V. Signaling pathways in ischemic preconditioning. Heart Fail. Rev. 12, 181–188 (2007).
Salazar, N. C., Chen, J. & Rockman, H. A. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim. Biophys. Acta 1768, 1006–1018 (2007).
Bak, M. I. & Ingwall, J. S. Acidosis during ischemia promotes adenosine triphosphate resynthesis in postischemic rat heart. In vivo regulation of 5′-nucleotidase. J. Clin. Invest. 93, 40–49 (1994).
Corriden, R. & Insel, P. A. Basal release of ATP: an autocrine–paracrine mechanism for cell regulation. Sci. Signal. 3, re1 (2010).
Eltzschig, H. K. et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 (2003).
Burnstock, G. Purinergic signaling in the cardiovascular system. Circ. Res. 120, 207–228 (2017).
Li, S. et al. Cardiomyocytes disrupt pyrimidine biosynthesis in nonmyocytes to regulate heart repair. J. Clin. Invest. 132, e149711 (2022).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Canto, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Horton, J. L. et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2, e84897 (2016).
Lee, C. F. et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134, 883–894 (2016).
Diguet, N. et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273 (2018).
Emily Flam, C. J. et al. Integrated landscape of cardiac metabolism in end-stage human nonischemic dilated cardiomyopathy. Nat. Cardiovasc. Res. 1, 817–829 (2022).
Martin, A. S. et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight 2, e93885 (2017).
Davidson, M. T. et al. Extreme acetylation of the cardiac mitochondrial proteome does not promote heart failure. Circ. Res. 127, 1094–1108 (2020).
Walker, M. A. et al. Acetylation of muscle creatine kinase negatively impacts high-energy phosphotransfer in heart failure. JCI Insight 6, e144301 (2021).
Walker, M. A. & Tian, R. Raising NAD in heart failure: time to translate? Circulation 137, 2274–2277 (2018).
Walker, M. A. & Tian, R. NAD(H) in mitochondrial energy transduction: implications for health and disease. Curr. Opin. Physiol. 3, 101–109 (2018).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 (2020).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020). A comprehensive evaluation of human cardiac fuel use, using arterial and coronary sinus blood sampling and quantifying global metabolites with mass spectrometry.
Lombardi, A. A. et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat. Commun. 10, 4509 (2019).
Gibb, A. A. et al. Glutaminolysis is essential for myofibroblast persistence and in vivo targeting reverses fibrosis and cardiac dysfunction in heart failure. Circulation 145, 1625–1628 (2022).
Gibb, A. A., Ploesch, T., Arany, Z. & Elrod, J. W. Genetic deletion of cardiomyocyte glutamine synthetase in a model of HF hints at cardiomyocyte to fibroblast metabolic crosstalk mediating fibrosis. Circulation 144, A14413 (2021).
Sun, H. et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133, 2038–2049 (2016).
Lai, L. et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ. Heart Fail. 7, 1022–1031 (2014).
Wang, W. et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 311, H1160–H1169 (2016).
Li, T. et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia–reperfusion injury. Cell Metab. 25, 374–385 (2017).
Chen, M. et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J. Am. Heart Assoc. 8, e011625 (2019).
Walejko, J. M. et al. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat. Commun. 12, 1680 (2021).
Murashige, D. et al. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab. 34, 1749–1764 (2022).
Previs, M. J. et al. Defects in the proteome and metabolome in human hypertrophic cardiomyopathy. Circ. Heart Fail. 15, e009521 (2022).
Jr Bedi, K. C. et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133, 706–716 (2016).
Montaigne, D., Butruille, L. & Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 18, 809–823 (2021).
Madrazo, J. A. & Kelly, D. P. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J. Mol. Cell. Cardiol. 44, 968–975 (2008).
Karamanlidis, G. et al. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 106, 1541–1548 (2010).
Levelt, E. et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65, 44–52 (2016).
Rijzewijk, L. J. et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 52, 1793–1799 (2008).
Leggat, J., Bidault, G. & Vidal-Puig, A. Lipotoxicity: a driver of heart failure with preserved ejection fraction? Clin. Sci. 135, 2265–2283 (2021).
Lehman, J. J. & Kelly, D. P. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin. Exp. Pharmacol. Physiol. 29, 339–345 (2002).
Riquelme, C. A. et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 334, 528–531 (2012). Dramatic demonstration of the ability of circulating metabolites, in this case, fatty acids, to promote cardiac hypertrophy, in this case, in the Burmese python of a once-in-a-month meal.
Linder, M. E. & Deschenes, R. J. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 (2007).
Essandoh, K., Philippe, J. M., Jenkins, P. M. & Brody, M. J. Palmitoylation: a fatty regulator of myocardial electrophysiology. Front. Physiol. 11, 108 (2020).
Schulze, P. C., Drosatos, K. & Goldberg, I. J. Lipid use and misuse by the heart. Circ. Res. 118, 1736–1751 (2016).
Harada, M. et al. Diacylglycerol kinase ζ attenuates pressure overload-induced cardiac hypertrophy. Circ. J. 71, 276–282 (2007).
Chokshi, A. et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125, 2844–2853 (2012).
Hadas, Y. et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation 141, 916–930 (2020).
Ji, R. et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2, e82922 (2017).
Park, T. S. et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112 (2008).
Means, C. K. & Brown, J. H. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200 (2009).
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S. & Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 (2010). Definitive review on fatty acid metabolism in the heart.
Choi, R. H., Tatum, S. M., Symons, J. D., Summers, S. A. & Holland, W. L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 18, 701–711 (2021).
Pinckard, K. M. et al. A novel endocrine role for the BAT-released lipokine 12,13-diHOME to mediate cardiac function. Circulation 143, 145–159 (2021). Identification of a new modified fatty acid, diHOME, released by adipose tissue, that contributes to mediating the cardiac response to exercise.
Stanford, K. I. et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27, 1111–1120 (2018).
Puchalska, P. & Crawford, P. A. Metabolic and signaling roles of ketone bodies in health and disease. Annu. Rev. Nutr. 41, 49–77 (2021).
Nielsen, R. et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 139, 2129–2141 (2019).
Byrne, N. J. et al. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ. Heart Fail. 13, e006573 (2020).
Newman, J. C. & Verdin, E. β-hydroxybutyrate: a signaling metabolite. Annu. Rev. Nutr. 37, 51–76 (2017).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011).
Rezq, S. & Abdel-Rahman, A. A. Central GPR109A activation mediates glutamate-dependent pressor response in conscious rats. J. Pharmacol. Exp. Ther. 356, 456–465 (2016).
Deng, Y. et al. Targeting mitochondria–inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ. Res. 128, 232–245 (2021).
Byrne, N. J. et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ. Heart Fail. 13, e006277 (2020).
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Cowie, M. R. & Fisher, M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 7, 761–772 (2020).
Philippaert, K. et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation 143, 2188–2204 (2021).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Chong, D. et al. Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming. Cell Discov. 8, 106 (2022).
Wu, L. F. et al. Global profiling of protein lysine malonylation in mouse cardiac hypertrophy. J. Proteomics 266, 104667 (2022).
Orsak, T. et al. Revealing the allosterome: systematic identification of metabolite–protein interactions. Biochemistry 51, 225–232 (2012).
Hicks, K. G. et al. Protein–metabolite interactomics reveals novel regulation of carbohydrate metabolism. Preprint at bioRxiv https://doi.org/10.1101/2021.08.28.458030 (2021).
Duncan, K. D., Fyrestam, J. & Lanekoff, I. Advances in mass spectrometry based single-cell metabolomics. Analyst 144, 782–793 (2019).
Husted, A. S., Trauelsen, M., Rudenko, O., Hjorth, S. A. & Schwartz, T. W. GPCR-mediated signaling of metabolites. Cell Metab. 25, 777–796 (2017).
Hakak, Y., Shrestha, D., Goegel, M. C., Behan, D. P. & Chalmers, D. T. Global analysis of G-protein-coupled receptor signaling in human tissues. FEBS Lett. 550, 11–17 (2003).
Olenchock, B. A. et al. EGLN1 inhibition and rerouting of α-ketoglutarate suffice for remote ischemic protection. Cell 164, 884–895 (2016).
Wyant, G. A. et al. Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists. Science 377, 621–629 (2022). Identification of a tryptophan metabolite that mediates the ability of skeletal muscle ischemic episodes to protect subsequent cardiac ischemia, a phenomenon known as distal ischemic preconditioning.
Divorty, N., Milligan, G., Graham, D. & Nicklin, S. A. The orphan receptor GPR35 contributes to angiotensin II-induced hypertension and cardiac dysfunction in mice. Am. J. Hypertens. 31, 1049–1058 (2018).
Li, H. et al. Novel role of GPR35 (G-protein-coupled receptor 35) in the regulation of endothelial cell function and blood pressure. Hypertension 78, 816–830 (2021).
He, W. et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 (2004).
Aguiar, C. J. et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun. Signal. 12, 78 (2014).
Yang, L. et al. Triggering the succinate receptor GPR91 enhances pressure overload-induced right ventricular hypertrophy. Int. J. Clin. Exp. Pathol. 7, 5415–5428 (2014).
Keiran, N. et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 20, 581–592 (2019).
Serena, C. et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 12, 1642–1657 (2018).
Cuspidi, C., Rescaldani, M., Sala, C. & Grassi, G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J. Hypertens. 32, 16–25 (2014).
Briscoe, C. P. et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 (2003).
Umar, S. et al. Free fatty acid receptor G-protein-coupled receptor 40 mediates lipid emulsion-induced cardioprotection. Anesthesiology 129, 154–162 (2018).
Shen, Y. J., Shen, Y. C., Lee, W. S. & Yang, K. T. Methyl palmitate protects heart against ischemia/reperfusion-induced injury through G-protein coupled receptor 40-mediated activation of the PI3K/AKT pathway. Eur. J. Pharmacol. 905, 174183 (2021).
Hirasawa, A. et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 (2005).
Murphy, K. A. et al. Free fatty acid receptor 4 responds to endogenous fatty acids to protect the heart from pressure overload. Cardiovasc. Res. 118, 1061–1073 (2021).
Witkowski, M., Weeks, T. L. & Hazen, S. L. Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570 (2020).
Tang, T. W. H. et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 139, 647–659 (2019).
Pluznick, J. L. et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013).
Nemet, I. et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 180, 862–877 (2020).
Romano, K. A. et al. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ. Heart Fail. 16, e009972 (2023).
Pu, J. et al. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur. Heart J. 34, 1834–1845 (2013).
Wang, J., Xu, T. & Xu, M. Roles and mechanisms of TGR5 in the modulation of CD4+ T cell functions in myocardial infarction. J. Cardiovasc. Transl. Res. 15, 350–359 (2022).
Acknowledgements
Z.A. is supported by NIH HL152446.
Author information
Authors and Affiliations
Contributions
E.F. and Z.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Yibin Wang, Rong Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flam, E., Arany, Z. Metabolite signaling in the heart. Nat Cardiovasc Res 2, 504–516 (2023). https://doi.org/10.1038/s44161-023-00270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00270-6