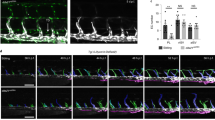
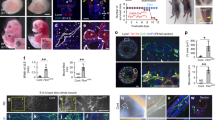
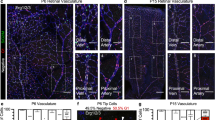
Abstract
VEGFR3 and its ligand VEGF-C are essential for lymphatic growth, but sinusoids in hematopoietic organs also express high levels of VEGFR3. Here we define a reciprocal VEGF-C/VEGFR3–CDH5 (VE-cadherin) signaling axis that controls both sinusoidal and lymphatic vessel growth. Loss of VEGF-C or VEGFR3 resulted in cutaneous edema, reduced fetal liver size and bloodless bone marrow due to impaired lymphatic and sinusoidal vessel growth, phenotypes shared with mice expressing membrane-retained VE-cadherin. In developing mice, loss of VE-cadherin rescued defects in sinusoidal and lymphatic growth conferred by loss of VEGFR3 but not loss of VEGF-C, findings explained by potentiated VEGF-C/VEGFR2 signaling. Mechanistically, VEGF-C/VEGFR3 signaling induces VE-cadherin endocytosis via SRC-mediated phosphorylation, whereas VE-cadherin prevents VEGFR3 endocytosis required for receptor signaling. These findings establish an essential role for VEGF-C/VEGFR3 signaling during sinusoidal vascular growth; identify VE-cadherin as a powerful negative regulator of VEGF-C signaling that acts through both VEGFR3 and VEGFR2 receptors; and suggest that negative regulation of VE-cadherin is required for effective VEGF-C/VEGFR3 signaling during growth of sinusoidal and lymphatic vessels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings in this study are included in the main article and associated files. Source Data are provided with this article. Transgenic mouse lines not available through public repositories are available from M.L.K. under a material transfer agreement with the University of Pennsylvania.
Code availability
The scripts used to analyze endothelial gene expression data can be found at: https://github.com/mhdominguez/Sung-NCVR-2022-scRNA/.
References
Griffin, C. T. & Gao, S. Building discontinuous liver sinusoidal vessels. J. Clin. Invest. 127, 790–792 (2017).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Hooper, A. T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009).
Ramasamy, S. K., Kusumbe, A. P., Wang, L. & Adams, R. H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA 92, 3566–3570 (1995).
Partanen, T. A. et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 14, 2087–2096 (2000).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Hogan, B. M. et al. ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 (2009).
Alders, M. et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 41, 1272–1274 (2009).
Le Guen, L. et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141, 1239–1249 (2014).
Saaristo, A., Karkkainen, M. J. & Alitalo, K. Insights into the molecular pathogenesis and targeted treatment of lymphedema. Ann. N. Y. Acad. Sci. 979, 94–110 (2002).
Fang, S. et al. Critical requirement of VEGF-C in transition to fetal erythropoiesis. Blood 128, 710–720 (2016).
Zou, Z. et al. The secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesis. Blood 121, 3228–3236 (2013).
Bui, H. M. et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Invest. 126, 2167–2180 (2016).
Dartsch, N., Schulte, D., Hagerling, R., Kiefer, F. & Vestweber, D. Fusing VE-cadherin to α-catenin impairs fetal liver hematopoiesis and lymph but not blood vessel formation. Mol. Cell. Biol. 34, 1634–1648 (2014).
Gordon, E. J. et al. The endothelial adaptor molecule TSAd is required for VEGF-induced angiogenic sprouting through junctional c-Src activation. Sci. Signal. 9, ra72 (2016).
Gavard, J. & Gutkind, J. S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223–1234 (2006).
Lampugnani, M. G., Orsenigo, F., Gagliani, M. C., Tacchetti, C. & Dejana, E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174, 593–604 (2006).
Hagerling, R. et al. Distinct roles of VE-cadherin for development and maintenance of specific lymph vessel beds. EMBO J. 37, e98271 (2018).
Si-Tayeb, K., Lemaigre, F. P. & Duncan, S. A. Organogenesis and development of the liver. Dev. Cell 18, 175–189 (2010).
Langen, U. H. et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. 19, 189–201 (2017).
Lorenz, L. et al. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature 562, 128–132 (2018).
Esser, S., Lampugnani, M. G., Corada, M., Dejana, E. & Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 111, 1853–1865 (1998).
Sun, Z. et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J. Exp. Med. 209, 1363–1377 (2012).
Grazia Lampugnani, M. et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161, 793–804 (2003).
Irrthum, A., Karkkainen, M. J., Devriendt, K., Alitalo, K. & Vikkula, M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 67, 295–301 (2000).
Karkkainen, M. J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Shin, M. et al. Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors. Development 143, 3785–3795 (2016).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Coso, S., Zeng, Y., Opeskin, K. & Williams, E. D. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS ONE 7, e39558 (2012).
Deng, Y., Zhang, X. & Simons, M. Molecular controls of lymphatic VEGFR3 signaling. Arterioscler. Thromb. Vasc. Biol. 35, 421–429 (2015).
Dellinger, M. T., Meadows, S. M., Wynne, K., Cleaver, O. & Brekken, R. A. Vascular endothelial growth factor receptor-2 promotes the development of the lymphatic vasculature. PLoS ONE 8, e74686 (2013).
Heinolainen, K. et al. VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ. Res. 120, 1414–1425 (2017).
Simons, M. An inside view: VEGF receptor trafficking and signaling. Physiology (Bethesda) 27, 213–222 (2012).
Wallez, Y. et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene 26, 1067–1077 (2007).
Li, X. et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 7, 11017 (2016).
Sawamiphak, S. et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465, 487–491 (2010).
Zhang, L. et al. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 20, 1319–1331 (2010).
Zhang, Y. et al. Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nat. Commun. 9, 1296 (2018).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Ding, B. S. et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 (2010).
Harris, N. R. et al. VE-cadherin is required for cardiac lymphatic maintenance and signaling. Circ. Res. 130, 5–23 (2022).
Tammela, T. et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 13, 1202–1213 (2011).
Karaman, S. et al. Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance. J. Exp. Med. 219, e20210565 (2022).
Kunnapuu, J. & Jeltsch, M. Outside in and brakes off for lymphatic growth. Sci. Signal. 14, eabj5058 (2021).
Herantis Pharma. Herantis announces inconclusive results from phase II study with lymfactin in breast cancer related lymphedema. https://herantis.com/press-releases/herantis-announces-inconclusive-results-from-phase-ii-study-with-lymfactin-in-breast-cancer-related-lymphedema/ (2021).
Stevenson Keller 4th, T. C. et al. Genetic blockade of lymphangiogenesis does not impair cardiac function after myocardial infarction. J. Clin. Invest. 131, e147070 (2021).
Schulte, D. et al. Stabilizing the VE-cadherin–catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 30, 4157–4170 (2011).
Yang, Y., Cha, B., Motawe, Z. Y., Srinivasan, R. S. & Scallan, J. P. VE-cadherin is required for lymphatic valve formation and maintenance. Cell Rep. 28, 2397–2412 (2019).
He, P. et al. The changing mouse embryo transcriptome at whole tissue and single-cell resolution. Nature 583, 760–767 (2020).
Wang, X. et al. Comparative analysis of cell lineage differentiation during hepatogenesis in humans and mice at the single-cell transcriptome level. Cell Res. 30, 1109–1126 (2020).
Liu, Y. et al. A specialized bone marrow microenvironment for fetal haematopoiesis. Nat. Commun. 13, 1327 (2022).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779 (2020).
Acknowledgements
We thank the members of the Kahn laboratory for their thoughtful comments and advice during this work. We thank the Cell & Developmental Biology Microscopy Core for microscopy support. This work was supported by NIH grants R01 DK123528 (M.L.K.), F30 HL158014 and T32 HL007439 (D.C.S.), T32 HL007483 (M.H.D.), T32 HL007150 (A.A.R.) and R01 HL142905 and R01 HL164825 (J.P.S.); American Heart Association postdoctoral fellowship number 836238 (X.C.); and Deutsche Forschungsgemeinschaft grants SFB1009 (A1) and SFB1348 (B1) (D.V.).
Author information
Authors and Affiliations
Contributions
D.C.S. designed and performed most of the mouse and tissue culture experiments. M.C. performed immunoblotting experiments. A.M. conducted initial hypothesis-generating experiments. S.G., M.H.D., X.C., A.A.R. and A.T.T. contributed to mouse genetic studies. R.P. contributed to tissue culture data analysis. J.Y. performed histological studies. P.M. and M.L. assisted with mouse handling and transfer. M.J., A.F.N., D.V. and J.P.S. contributed to data interpretation. J.P.S. generated and characterized the Cdh5 conditional allele. A.F.N. and D.V. generated and characterized the Cdh5α allele. D.C.S., A.M. and M.L.K. designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Christopher Kontos, Saravana Ramasamy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Extended Data Fig. 1 Efficient inducible deletion of VEGFR3 in sinusoidal and lymphatic endothelial cells.
a, Immunofluorescence staining of bone marrow for the sinusoidal endothelial marker Endomucin (red) and VEGFR3 (green) in Control and Cdh5CreERT2; Flt4fl/fl E18.5 mice. Control, n = 5. Cdh5CreERT2; Flt4fl/fl, n = 4. Scale bars = 100 μm. b, c, Immunofluorescence staining of the liver and skin for the sinusoidal/lymphatic endothelial marker LYVE1 (red) and VEGFR3 (green) in Control and Cdh5CreERT2; Flt4fl/fl E14.5 mice. Lack of VEGFR3 in Cdh5CreERT2; Flt4fl/fl embryos confirms efficiency of deletion induced by tamoxifen. Control, n = 7. Cdh5CreERT2; Flt4fl/fl, n = 6. Scale bars = 50 μm.
Extended Data Fig. 2 Sinusoidal endothelial cell proliferation in the mid-gestation liver occurs predominantly in the outer zone.
Immunofluorescence staining and quantification of a wildtype E14.5 liver for LYVE1 (red) and Ki67 (green) demonstrating the differences in proliferation, which is greater in the outer zone (OZ) relative to the central zone (CZ). n = 3 wildtype livers. Scale bar = 500 μm. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test. Data are shown as means ± S.D.
Extended Data Fig. 3 Membrane-retained VE-cadherin confers defects in fetal liver sinusoidal angiogenesis identical to loss of VEGFR3 function.
a, Whole embryos (16x magnification) from control and Cdh5α/α E14.5 mice. White dotted lines outline the liver contour, and the red arrowhead indicates severe edema. b, H&E staining of liver. Control, n = 11. Cdh5α/α, n = 7. Scale bars = 100 μm. c, Immunofluorescence staining of the liver for the sinusoidal marker LYVE1 (red) and proliferation marker Ki67 (green), and quantification of sinusoidal vascular area by zone (Control, n = 11. Cdh5α/α, n = 7) and percent Ki67+ (Control, n = 5. Cdh5α/α, n = 4) sinusoidal endothelial cells. Scale bars = 100 μm. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test. Data are shown as means ± S.D.
Extended Data Fig. 4 Efficient inducible deletion of VEGFR3 in sinusoidal endothelium.
a, Immunofluorescence staining of bone marrow for the sinusoidal endothelial marker Endomucin (red) and VEGFR3 (green) in Control, Cdh5CreERT2; Flt4fl/fl, and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ E18.5 mice. Control, n = 4. Cdh5CreERT2; Flt4fl/fl, n = 3. Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+, n = 4. Scale bars = 100 μm. b, Immunofluorescence staining of the liver for the sinusoidal endothelial marker LYVE1 (red) and VEGFR3 (green) in Control, Cdh5CreERT2; Flt4fl/fl, and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ E14.5 mice. Lack of VEGFR3 in Cdh5CreERT2; Flt4fl/fl and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ embryos confirms efficiency of deletion induced by tamoxifen. Control, n = 8. Cdh5CreERT2; Flt4fl/fl, n = 6. Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+, n = 4. Scale bars = 100 μm.
Extended Data Fig. 5 Loss of lymphatic growth in Cdh5α/α skin, efficient deletion of VEGFR3 in Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ mice, and loss of pERK1/2 signaling in Cdh5α/α bone marrow.
a, H&E and immunofluorescence staining for TER119 (green), LYVE1 (red), and the lymphatic nuclear marker PROX1 (gray) in skin sections from control and Cdh5α/α E14.5 embryos. White arrowheads indicate LYVE1+PROX1+ lymphatic endothelial cells, and double-headed arrow with ‘E’ indicates presence of edema. Quantification of number of lymphatic endothelial cells per area. Control, n = 5. Cdh5α/α, n = 4. Scale bars = 50 μm. b, Immunofluorescence staining of the skin for the lymphatic endothelial marker LYVE1 (red) and VEGFR3 (green) in Control, Cdh5CreERT2; Flt4fl/fl, and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ E14.5 mice. Lack of VEGFR3 in Cdh5CreERT2; Flt4fl/fl and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ embryos confirms efficiency of deletion induced by tamoxifen. Control, n = 8. Cdh5CreERT2; Flt4fl/fl, n = 6. Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+, n = 4. Scale bars = 50 μm. c, Immunofluorescence staining of bone marrow for Endomucin (red), TER119 (gray), and pERK1/2 (green) from Control and Cdh5α/α E18.5 mice, and quantification of percent pERK1/2+ sinusoidal endothelial cells. Yellow arrowheads indicate pERK1/2+ sinusoidal endothelial cells. Control, n = 6. Cdh5α/α, n = 4. Scale bars = 25 μm. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test. Data are shown as means±S.D.
Extended Data Fig. 6 VEGF-C, VEGF-CC156S, and VEGF-A differentially activate growth factor signals in cultured LECs.
a, Immunoblot analysis and quantification of pERK1/2 and pAKT expression in LECs treated with control or VEGF-C (100 ng/ml) supplemented media at various time points. n = 3 independent experiments per condition per time point. *P < 0.05. b, Immunoblot analysis and quantification of pERK1/2, pAKT, and pS6 expression in LECs treated with control, VEGF-C (100 ng/ml), VEGF-CC156S (50μg/ml), or VEGF-A (50 ng/ml) supplemented media for 15 minutes. n = 3 independent experiments per condition. c, Immunoblot analysis and quantification of VEGFR3 and VE-cadherin expression confirms efficiency of knockdown in response to siRNA treatment against VEGFR3 (siFLT4) and/or VE-cadherin (siCDH5). n = 4 independent experiments per condition. d, mRNA fold change by qRT-PCR in E14.5 Control and R26CreERT2; Vegfcfl/fl; Cdh5fl/+ embryos confirms efficiency of VEGF-C deletion. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test for a, b, and d and one-way ANOVA with Tukey’s test for multiple comparisons for c. Data are shown as means±S.D.
Extended Data Fig. 7 Expression of VEGFR2, pY1175 VEGFR2, and VE-cadherin in bone marrow sinusoids.
a, b, Immunofluorescence staining and quantification of bone marrow sections for the sinusoidal endothelial marker Endomucin (red) and VEGFR2 (green, a) or pY1175 VEGFR2 (green, b) in Control, Cdh5CreERT2; Flt4fl/fl, and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ E18.5 mice. Green arrowheads point to positive sinusoidal VEGFR2 or pY1175 VEGFR2 staining. Control, n = 4. Cdh5CreERT2; Flt4fl/fl, n = 3. Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+, n = 4. Scale bars = 25 μm. c, Immunofluorescence staining of the bone marrow for the sinusoidal endothelial marker Endomucin (red) and VE-cadherin (green) in Control, Cdh5CreERT2; Flt4fl/fl, and Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+ E18.5 mice. Control, n = 4. Cdh5CreERT2; Flt4fl/fl, n = 3. Cdh5CreERT2; Flt4fl/fl; Cdh5fl/+, n = 4. Scale bars = 100 μm. Statistical analysis was performed using one-way ANOVA with Tukey’s test for multiple comparisons. Data are shown as means ± S.D.
Extended Data Fig. 8 scRNA-seq analysis of VE-cadherin, VEGFR2, and VEGFR3 in skin BECs and LECs.
a, Analysis of single cell RNA-seq on the mouse embryonic forelimb and skin by He et al. Endothelial clusters were identified by expression of multiple BEC- and LEC-specific markers. b, Violin plots of Cdh5, Kdr, and Flt4 expression in BECs and LECs demonstrate lower levels of Cdh5, similar levels of Kdr, and higher expression of Flt4 in LECs. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test.
Extended Data Fig. 9 scRNA-seq analysis of VE-cadherin, VEGFR2, and VEGFR3 in liver and bone marrow BECs and SECs.
a, Analysis of single cell RNA-seq on the fetal mouse liver (E11.5-17.5) by Wang et al. Endothelial clusters were identified by expression of multiple BEC- and liver SEC-specific markers. b, Violin plots of Cdh5, Kdr, and Flt4 expression in BECs and liver SECs demonstrates similar levels of Cdh5 and higher levels of Kdr and Flt4 in liver SECs. c, Analysis of single cell RNA-seq on the fetal mouse bone marrow (E18.5) by Liu et al. Endothelial clusters were identified by expression of multiple BEC- and bone marrow SEC-specific markers. d, Violin plots of Cdh5, Kdr, and Flt4 expression in BECs and bone marrow SECs demonstrates similar levels of Cdh5 and higher levels of Kdr and Flt4 in bone marrow SECs. e, Table summarizing expression patterns of VEGFR2 and VEGFR3 based on gene and protein expression analysis. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test.
Extended Data Fig. 10 Reciprocal VEGF-C/VE-cadherin regulation in sinusoidal and lymphatic vessels of wild-type and mutant mice.
The schematics shown describe predicted SEC and LEC signaling responses in the loss and gain of function conditions examined in this study. They incorporate both in vivo and in vitro findings and are speculations based on the available data. a, In wildtype embryos, VEGF-C/VEGFR3 promotes sinusoidal and lymphatic growth through reciprocal VEGFR3-VE-cadherin regulation. Sinusoids highly express VEGFR3 and VEGFR2 (VEGFR3+++, VEGFR2+++) in contrast to lymphatics that highly express VEGFR3 but less VEGFR2 (VEGFR3+++, VEGFR2++). b, c, Loss of VEGFR3 (Cdh5CreERT2; Flt4fl/fl) or VEGF-C (R26CreERT2; Vegfcfl/fl) decreases growth factor signaling due to both loss of ligand-activated signals and gain of negative regulation by unopposed VE-cadherin, resulting in decreased sinusoidal and lymphatic growth. d, Gain of VE-cadherin (Cdh5α/α) negatively regulates VEGFR3 (and perhaps also VEGFR2 in sinusoids), resulting in reduced growth factor signaling and loss of sinusoidal and lymphatic growth. e, Loss of VE-cadherin compensates for loss of VEGFR3 by potentiating VEGFR2 signaling in response to available VEGF-C (in LECs) and VEGF-C + VEGF-A (in SECs). Complete rescue of sinusoidal growth may reflect the fact that SECs are VEGFR2+++, while incomplete rescue of lymphatic growth may reflect the fact that lymphatics are VEGFR2++. f, Loss of VE-cadherin is unable to compensate for loss of VEGF-C signaling in VEGFR2++ LECs but partially compensates for loss of VEGF-C by potentiating VEGF-A-VEGFR2 signaling in VEGFR2+++ SECs. The proposed role of VEGF-A is inferred from our and prior studies.
Supplementary information
Source data
Source Data Fig. 1
Raw and statistical source data in Fig. 1
Source Data Fig. 2
Raw and statistical source data in Fig. 2
Source Data Fig. 3
Raw and statistical source data in Fig. 3
Source Data Fig. 4
Raw and statistical source data in Fig. 4
Source Data Fig. 5
Raw and statistical source data in Fig. 5
Source Data Fig. 6
Raw and statistical source data in Fig. 6
Source Data Fig. 6
Unprocessed western blots
Source Data Fig. 7
Raw and statistical source data in Fig. 7
Source Data Fig. 7
Unprocessed western blots
Source Data Fig. 8
Raw and statistical source data in Fig. 8
Source Data Fig. 8
Unprocessed western blots
Source Data Extended Data Fig. 2
Raw and statistical source data in Extended Data Fig. 2
Source Data Extended Data Fig. 3
Raw and statistical source data in Extended Data Fig. 3
Source Data Extended Data Fig. 5
Raw and statistical source data in Extended Data Fig. 5
Source Data Extended Data Fig. 6
Raw and statistical source data in Extended Data Fig. 6
Source Data Extended Data Fig. 6
Unprocessed western blots
Source Data Extended Data Fig. 7
Raw and statistical source data in Extended Data Fig. 7
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sung, D.C., Chen, M., Dominguez, M.H. et al. Sinusoidal and lymphatic vessel growth is controlled by reciprocal VEGF-C–CDH5 inhibition. Nat Cardiovasc Res 1, 1006–1021 (2022). https://doi.org/10.1038/s44161-022-00147-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00147-0
This article is cited by
-
VEGF-C and VE-cadherin: balancing sinusoidal and lymphatic angiogenesis
Nature Cardiovascular Research (2022)