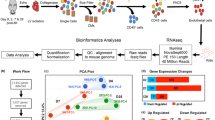
Abstract
Myocardial infarction (MI) is a leading cause of death worldwide, largely because efficient interventions to restore cardiac function after MI are currently lacking. Here, we characterize vascular aberrancies induced by MI and propose to target acquired endothelial cell (EC) changes to normalize vessels and promote cardiac repair after MI. Single-cell transcriptome analyses of MI-associated ECs indicates that ECs acquire mesenchymal gene signatures that result in phenotypic and functional changes and lead to vessel abnormalities. We identify a platelet-derived growth factor (PDGF)–nuclear factor κB (NF-κB)–hypoxia-inducible factor 1-α (HIF-1α) axis that induces Snail expression and mesenchymal phenotypes in ECs under hypoxia, altogether causing aberrant vascularization. EC-specific knockout of platelet-derived growth factor receptor beta (PDGFR-β), pharmacological PDGFR inhibition or nanoparticle-based targeted PDGFR-β small interfering RNA delivery in mice attenuates vascular abnormalities in the infarcted tissue and improves cardiac repair after MI. These findings illustrate a mechanism controlling aberrant neovascularization after ischemia and suggest that targeting PDGF/Snail-mediated endothelial plasticity may offer opportunities for normalizing vasculature and treating ischemic heart diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq data have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus under accession no. GSE163772, while the single-cell and single-nuclei RNA-seq analysis of mouse cardiac tissues with MI have been deposited in the same publicly available database under the accession nos. GSE163956 and GSE193290, respectively. The data supporting the findings of this study are available within the paper and its supplementary information. Source data are provided with this paper.
References
Ware, J. A. & Simons, M. Angiogenesis in ischemic heart disease. Nat. Med. 3, 158–164 (1997).
Harada, K. et al. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am. J. Physiol. 270, H1791–H1802 (1996).
Banai, S. et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 89, 2183–2189 (1994).
Henry, T. D. et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107, 1359–1365 (2003).
Stewart, D. J. et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 17, 1109–1115 (2009).
Rajagopalan, S. et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 108, 1933–1938 (2003).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 (2011).
Kovacic, J. C., Mercader, N., Torres, M., Boehm, M. & Fuster, V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125, 1795–1808 (2012).
Piera-Velazquez, S. & Jimenez, S. A. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 99, 1281–1324 (2019).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Maddaluno, L. et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498, 492–496 (2013).
Manavski, Y. et al. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ. Res. 122, 670–677 (2018).
Li, Z. et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur. Heart J. 40, 2507–2520 (2019).
Tombor, L. S. et al. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction. Nat. Commun. 12, 681 (2021).
Huang, M. et al. c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Invest. 126, 1801–1814 (2016).
Fan, Y. Vascular detransformation for cancer therapy. Trends Cancer 5, 460–463 (2019).
De Palma, M. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 (2008).
Acloque, H., Adams, M. S., Fishwick, K., Bronner-Fraser, M. & Nieto, M. A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438–1449 (2009).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Liu, T. et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 9, 3439 (2018).
Khan, O. F. et al. Endothelial siRNA delivery in nonhuman primates using ionizable low-molecular weight polymeric nanoparticles. Sci. Adv. 4, eaar8409 (2018).
Krohn-Grimberghe, M. et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat. Biomed. Eng. 4, 1076–1089 (2020).
Hoeben, A. et al. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56, 549–580 (2004).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Oladipupo, S. et al. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl Acad. Sci. USA 108, 13264–13269 (2011).
Nagy, J. A., Dvorak, A. M. & Dvorak, H. F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2, 251–275 (2007).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Potenta, S., Zeisberg, E. & Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 99, 1375–1379 (2008).
Chen, P.-Y. et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Invest. 125, 4514–4528 (2015).
Dejana, E., Hirschi, K. K. & Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 8, 14361 (2017).
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3, 155–166 (2002).
Bachelder, R. E., Yoon, S.-O., Franci, C., de Herreros, A. G. & Mercurio, A. M. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial–mesenchymal transition. J. Cell Biol. 168, 29–33 (2005).
Pires, B. R. B. et al. NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS ONE 12, e0169622 (2017).
Zhu, Y. et al. HIF-1α regulates EMT via the Snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J. Cell. Mol. Med. 20, 688–697 (2016).
Zhang, L. et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer 13, 108 (2013).
Xu, X. et al. Snail is a direct target of hypoxia-inducible factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J. Biol. Chem. 290, 16653–16664 (2015).
Kokudo, T. et al. Snail is required for TGFβ-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J. Cell Sci. 121, 3317–3324 (2008).
Mahmoud, M. M. et al. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci. Rep. 7, 3375 (2017).
Cheng, J.-C., Chang, H.-M. & Leung, P. C. K. Transforming growth factor-β1 inhibits trophoblast cell invasion by inducing Snail-mediated down-regulation of vascular endothelial-cadherin protein. J. Biol. Chem. 288, 33181–33192 (2013).
Lopez, D., Niu, G., Huber, P. & Carter, W. B. Tumor-induced upregulation of Twist, Snail, and Slug represses the activity of the human VE-cadherin promoter. Arch. Biochem. Biophys. 482, 77–82 (2009).
Ishikawa, F. et al. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature 338, 557–562 (1989).
Benjamin, L. E., Hemo, I. & Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125, 1591–1598 (1998).
Battegay, E. J., Rupp, J., Iruela-Arispe, L., Sage, E. H. & Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 125, 917–928 (1994).
Liu, C. et al. Platelet-derived growth factor blockade on cardiac remodeling following infarction. Mol. Cell. Biochem. 397, 295–304 (2014).
Zymek, P. et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J. Am. Coll. Cardiol. 48, 2315–2323 (2006).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Gong, Y., Zhao, Y., Li, Y., Fan, Y. & Hoover-Plow, J. Plasminogen regulates cardiac repair after myocardial infarction through its noncanonical function in stem cell homing to the infarcted heart. J. Am. Coll. Cardiol. 63, 2862–2872 (2014).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16, 233–270 (2015).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Liu, T. et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 78, 6632–6642 (2018).
Yang, B. et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958 (2014).
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
Acknowledgements
We thank J. Eberwine (Penn Pharmacology), J. Schug (Penn Next-Generation Sequencing Core), A. Stout (Penn CDB Microscope Core) and E. Blankemeyer (Penn Small Animal Imaging Core) for assistance with RNA linear amplification, scRNA-seq, light sheet fluorescence imaging and SPECT analysis, respectively. This work was supported in part by an American Heart Association (AHA) Innovative Project Award no. IPA34170252 (to Y.F.), National Institutes of Health (NIH) grant nos. R01HL155198 (to Y.F. and Y.G.) and R01NS094533, R01NS106108 and R01CA241501 (to Y.F.), American Association for Cancer Research (AACR) Judah Folkman Award (to Y.F.), AHA Scientist Development grant no. SDG9050018 and grant-in-Aid no. GRNT3365002 (to Y.G.) and AHA Predoctoral Fellowship (to D.Z.). L.P. is supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program (nos. W81XWH2010042 and W81XWH2010089). M.J.M. acknowledges support from a Burroughs Wellcome Fund Career Award at the Scientific Interface, an NIH Director’s New Innovator Award (no. DP2 TR002776), NIH grant nos. R01CA241661, R37CA244911 and R01DK123049, an Abramson Cancer Center-School of Engineering and Applied Sciences Discovery Grant (no. P30CA016520) and an AACR-Bayer Innovation and Discovery Grant (no. 18-80-44-MITC).
Author information
Authors and Affiliations
Contributions
M.H., F.Y., D.Z. and M.L. designed, performed and analyzed the experiments. D.Z., L.Z. and L.Q. contributed to the scRNA-seq studies. M.L. and S.V.S. contributed to the echocardiogram analysis. H.D. helped tissue histology analysis. R.E. and M.J.M. contributed to nanoparticle production. L.P. helped with the single-nucleus analysis. D.J.R. contributed to the experimental design and discussion. Y.G. and M.L. performed the MI surgery. Y.F. and Y.G. cosupervised the experiments and wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
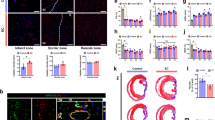
Extended Data Fig. 1 Aberrant vascularization characterizes infarcted cardiac tissue.
Mice were subjected to MI surgery or sham operation. Hearts were excised 3 weeks after surgery. Tissue sections were immunostained with (a) anti-CD31 antibody, (b) anti-NG2 and anti-CD31 antibodies, or (c) anti-collagen IV and anti-CD31 antibodies. a, Representative images in normal tissue and infarct zone are shown (n = 3 mice). Bars indicate 100 µm. b,c, Left, representative images in normal tissue and infarct zone are shown. Right, quantified results (mean ± SEM, n = 3 mice). Statistical analysis by unpaired two-tailed Students’ t test. Bars indicate 100 µm.
Extended Data Fig. 2 Cardiomyocyte-conditioned medium induces FSP-1 and α-SMA expression in ECs.
Mouse cardiac-conditioned medium (CCM) were harvested from primary mouse cardiomyocytes cultured under hypoxia (1% O2). Mouse cardiac microvascular ECs were treated with CCM under normoxia or hypoxia for 2 days. Cell lysates were immunoblotted. These experiments were repeated independently twice with similar results.
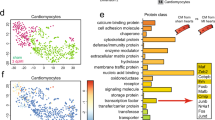
Extended Data Fig. 3 Expression of mesenchymal genes in MI-associated ECs.
MI was induced in wild-type mice. Three weeks after MI induction, ECs were isolated from normal LV and MI tissues. RNA was extracted and subjected to RT-PCR analysis (mean ± SEM, n = 5-7 mice, specific n indicated in the graphs). Statistical analysis by unpaired two-tailed Students’ t test. Expression of (a) S100A4 (FSP-1) and Acta2 (α-SMA), and (b) Snai1 (Snail), Snai2 (Slug), and Twist1 was normalized with GAPDH expression levels.
Extended Data Fig. 4 PDGF-AB induces expression of mesenchymal proteins in ECs.
Human cardiac microvascular ECs were treated with 100 ng/ml SCF, PDGF-AB, or HGF for 2 days under hypoxia. Cell lysates were immunoblotted. These experiments were repeated independently twice with similar results.
Extended Data Fig. 5 PDGFR-β knockout inhibits PDGF-AB-induced phosphorylation of Erk1 and Akt1 in ECs and does not affect vascular density in normal hearts.
a, Aortic ECs were isolated from tamoxifen-treated Cdh5-CreERT2;Pdgfrbfl/fl (PDGFR-β-ΔEC) and Pdgfrbfl/fl (control) mice. Cells were treated with 100 ng/ml PDGF-AB for 10 min, followed by immunoblot analysis. These experiments were repeated independently twice with similar results. b,Left ventricles were collected from PDGFR-β-ΔEC and control mice. Cardiac sections were stained with anti-CD31 antibody and analyzed by immunofluorescence. Left, representative images are shown. Scale bar: 200 μm. Right, quantified results (mean ± SEM, n = 6 mice). Statistical analysis by unpaired two-tailed Students’ t test.
Extended Data Fig. 6 PDGFR-β knockout in ECs does not increase vascular density in infarct zone and border zone.
MI was induced in control and PDGFR-β-ΔEC mice. (a,b) 4 or (c) 8 weeks after MI induction, cardiac tissues were collected. Sections were stained with anti-collagen I and anti-CD31 antibodies, followed by immunofluorescence analysis. a, Representative images are shown (n = 6 mice). Scale bar: 100 μm. b,c, Vascular density was quantified (mean ± SEM). b, n = 3-4 mice, specific n indicated in the graphs. c, n = 6 mice. Statistical analysis by two-way ANOVA Fisher’s test.
Extended Data Fig. 7 PDGFR-β knockout reduces vascular density and inhibits Ki67, Snail and FSP-1 expression in MI- associated ECs.
MI was induced in WT and PDGFR-β-ΔEC mice. Hearts were excised 3 weeks after surgery. MI tissue sections were immunostained with (a-c) anti-Ki67 and anti-CD31, (d) anti-vWF and anti-Snail, or (e) anti-CD31 and anti-FSP-1 antibodies. a, Representative immunofluorescence images in infarct zone are shown (n = 3 mice). Scale bar: 100 μm. b,c, quantified results (mean ± SEM, n = 3 mice). b, Density of CD31+ cells. AU, arbitrary unit. c, Ki67 expression in CD31+ cells. Statistical analysis by unpaired two-tailed Students’ t test. d,e, Representative immunofluorescence images in infarct zone are shown (n = 5 mice). Scale bar: 100 μm.
Extended Data Fig. 8 PDGFR-β knockout reduces Snail expression in MI-associated ECs.
MI was induced in WT and PDGFR-β-ΔEC mice. (a,b) 14 and (c,d) 28 days after MI induction, MI tissue sections were collected and subjected to single-nuclei RNA sequencing analysis (total = 4 mice). Uniform manifold approximation and projection (UMAP) analysis of transcriptome gene signature in ECs. (a,c) UMAP analysis of ECs. (b,d) Expression of Snail, Slug, and Ki67 in ECs.
Extended Data Fig. 9 PDGFR-β knockout in ECs alters expression of metabolism-associated genes in myocytes.
MI was induced in WT and PDGFR-β-ΔEC mice. 14 and 28 days after MI induction, MI tissue sections were collected and subjected to single-nuclei RNA sequencing analysis (total = 4 mice). Expression of metabolism-associated genes were analyzed. a, Heatmap of mapped genes. b, Global changes in gene expression at days 14 and 28 after MI induction.
Extended Data Fig. 10 PDGFR inhibition improves cardiac function recovery and promotes tissue repair after MI.
MI was induced in mice, followed by administration with saline or 15 mg/kg crenolanib. a,b, Cardiac function was analyzed by echocardiogram (n = 11 mice). Representative images are shown. a, Long-axis echocardiogram analysis. Scale bar: 2 mm. b, M mode echocardiogram analysis. Scale bar: 2 mm. Arrows indicate myocardial anterior walls. c,d, Heart tissues were harvested 3 weeks after MI induction. Cardiac sections were stained with Masson’s trichrome stain. c, Representative images are shown (n = 4 mice). Scale bar: 500 μm. Arrows indicate epicardium. d, Quantified cardiomyocyte area (mean ± SEM, n = 4-5 mice). Statistical analysis by unpaired two-tailed Students’ t test. e, Heart tissues were harvested 3 weeks after MI induction. Cardiac sections were stained with an anti-CD31 antibody and analyzed by immunofluorescence imaging. Vascular density was quantified (mean ± SEM, n = 5 mice). Statistical analysis by two-way ANOVA.
Supplementary information
Supplementary Video 1.
MI was induced in control and PDGFR-β-ΔEC mice. Mice underwent echocardiography analysis at days 14 and 28 after MI.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, M., Yang, F., Zhang, D. et al. Endothelial plasticity drives aberrant vascularization and impedes cardiac repair after myocardial infarction. Nat Cardiovasc Res 1, 372–388 (2022). https://doi.org/10.1038/s44161-022-00047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00047-3
This article is cited by
-
Spatial analysis of tissue immunity and vascularity by light sheet fluorescence microscopy
Nature Protocols (2024)