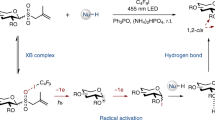
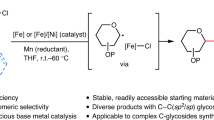
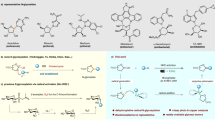
Abstract
State-of-the-art glycosylation methods primarily rely on ionic reactions of heteroatomic nucleophiles with electrophilic glycosyl oxocarbenium intermediates. Although such ionic glycosylation strategies can effectively form O-glycosides, their use in N-glycoside synthesis is often plagued by the subdued reactivity of N-nucleophiles under the acidic reaction conditions required for glycosyl donor activation. Exploration of the reactivity of glycosyl radical intermediates has begun to offer new glycosylation pathways. However, despite recent progress in radical-mediated synthesis of C-glycosides, harnessing the reactivity of glycosyl radicals for the generation of canonical O- or N-glycosides remains elusive. Here we report the development of a glycosyl radical-mediated N-glycosylation reaction using readily accessible glycosyl sulfone donors and N-nucleophiles under mild copper-catalysed, photoredox-promoted conditions. The method is efficient, selective, redox neutral and broadly applicable, enabling ready access to a variety of complex N-glycosides and nucleosides in a streamlined fashion. Importantly, the present system tolerates the presence of water and offers unique chemoselectivity, allowing selective reaction of NH sites over hydroxyl groups that would otherwise pose challenges in conventional ionic N-glycosylation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or Supplementary Information. Crystallographic data for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition no. CCDC 2266734 (51). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
Shivatare, S. S., Shivatare, V. S. & Wong, C.-H. Glycoconjugates: synthesis, functional studies, and therapeutic developments. Chem. Rev. 122, 15603–15671 (2022).
Yang, Y., Zhang, X. & Yu, B. O-glycosylation methods in the total synthesis of complex natural glycosides. Nat. Prod. Rep. 32, 1331–1355 (2015).
Bokor, É. et al. C-glycopyranosyl arenes and hetarenes: synthetic methods and bioactivity focused on antidiabetic potential. Chem. Rev. 117, 1687–1764 (2017).
Hsu, C.-H., Hung, S.-C., Wu, C.-Y. & Wong, C.-H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. Engl. 50, 11872–11923 (2011).
Sangwan, R., Khanam, A. & Mandal, P. K. An overview on the chemical N-functionalization of sugars and formation of N-glycosides. Eur. J. Org. Chem. 2020, 5949–5977 (2020).
Jordheim, L. P., Durantel, D., Zoulim, F. & Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug. Discov. 12, 447–464 (2013).
Zhang, Q., Sun, J., Zhu, Y., Zhang, F. & Yu, B. An efficient approach to the synthesis of nucleosides: gold(I)-catalyzed N-glycosylation of pyrimidines and purines with glycosyl ortho-alkynyl benzoates. Angew. Chem. Int. Ed. Engl. 50, 4933–4936 (2011).
Stanley P. et al. in Essentials of Glycobiology 4th edn (eds Varki, A. et al.) Ch. 9 (Cold Spring Harbor Laboratory Press, 2022).
Chen, Z., Sato, S., Geng, Y., Zhang, J. & Liu, H.-W. Identification of the early steps in herbicidin biosynthesis reveals an atypical mechanism of c-glycosylation. J. Am. Chem. Soc. 144, 15653–15661 (2022).
Loustaud-Ratti, V. et al. Ribavirin: past, present and future. World J. Hepatol. 8, 123–130 (2016).
Staudacher, E., Van Damme, E. J. M. & Smagghe, G. Glycosylation—the most diverse post-translational modification. Biomolecules 12, 1313 (2022).
Chakraborty, S., Mishra, B., Das, P. R., Pasari, S. & Hotha, S. Synthesis of N-glycosides by silver-assisted gold catalysis. Angew. Chem. Int. Ed. Engl. 62, e202214167 (2023).
Ding, F., William, R. & Liu, X.-W. Ferrier-type N-glycosylation: synthesis of N-glycosides of enone sugars. J. Org. Chem. 78, 1293–1299 (2013).
Li, P. et al. Glycosyl ortho-(1-phenylvinyl)benzoates versatile glycosyl donors for highly efficient synthesis of both O-glycosides and nucleosides. Nat. Commun. 11, 405 (2020).
Liu, R. et al. Synthesis of nucleosides and deoxynucleosides via gold(I)-catalyzed N-glycosylation of glycosyl (Z)-ynenoates. Org. Lett. 24, 9479–9484 (2022).
Nielsen, M. M. & Pedersen, C. M. Catalytic glycosylations in oligosaccharide synthesis. Chem. Rev. 118, 8285–8358 (2018).
Panza, M., Pistorio, S. G., Stine, K. J. & Demchenko, A. V. Automated chemical oligosaccharide synthesis: novel approach to traditional challenges. Chem. Rev. 118, 8105–8150 (2018).
Kobayashi, Y., Nakatsuji, Y., Li, S., Tsuzuki, S. & Takemoto, T. Direct N-glycofunctionalization of amides with glycosyl trichloroacetimidate by thiourea/halogen bond donor co-catalysis. Angew. Chem. Int. Ed. Engl. 57, 3646–3650 (2018).
An, S. et al. Palladium-catalyzed O- and N-glycosylation with glycosyl chlorides. CCS Chem. 3, 1821–1829 (2021).
Toshima, K. & Tasuta, K. Recent progress in O-glycosylation methods and its application to natural products synthesis. Chem. Rev. 93, 1503–1531 (1993).
Mukherjee, M. M., Ghosh, R. & Hanover, J. A. Recent advances in stereoselective chemical O-glycosylation reactions. Front. Mol. Biosci. 9, 896187 (2022).
Chen, A. et al. Recent advances in glycosylation involving novel anomeric radical precursors. J. Carbohydr. Chem. 40, 361–400 (2021).
Xu, L.-Y., Fan, N.-L. & Hu, X.-G. Recent development in the synthesis of C-glycosides involving glycosyl radicals. Org. Biomol. Chem. 18, 5095–5109 (2020).
Jiang, Y., Zhang, Y., Lee, B. C. & Koh, M. J. Diversification of glycosyl compounds via glycosyl radicals. Angew. Chem. Int. Ed. Engl. 62, e202305138 (2023).
Shang, W. & Niu, D. Radical pathway glycosylation empowered by bench-stable glycosyl donors. Acc. Chem. Res. 56, 2473–2488 (2023).
Chen, A., Yang, B., Zhou, Z. & Zhu, F. Recent advances in transition-metal-catalyzed glycosyl cross-coupling reactions. Chem. Catal. 2, 3430–3470 (2022).
Wei, Y., Lin, L. Q. H., Lee, B. C. & Koh, M. J. Recent advances in first-row transition metal-catalyzed reductive coupling reactions for π-bond functionalization and C-glycosylation. Acc. Chem. Res. 56, 3292–3312 (2023).
Wang, Q. et al. Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones. Nat. Synth. 1, 967–974 (2022).
Jiang, Y., Wang, Q., Zhang, X. & Koh, M. J. Synthesis of C-glycosides by Ti-catalyzed stereoselective glycosyl radical functionalization. Chem 7, 3377–3392 (2021).
Xu, S. et al. Generation and use of glycosyl radicals under acidic conditions: glycosyl sulfinates as precursors. Angew. Chem. Int. Ed. Engl. 62, e202218303 (2023).
Shang, W. et al. Generation of glycosyl radicals from glycosyl sulfoxides and its use in the synthesis of C-linked glycoconjugates. Angew. Chem. Int. Ed. Engl. 60, 385–390 (2021).
Nagatomo, M. & Inoue, M. Convergent assembly of highly oxygenated natural products enabled by intermolecular radical reactions. Acc. Chem. Res. 54, 595–604 (2021).
Masuda, K., Nagatomo, M. & Inoue, M. Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions. Nat. Chem. 9, 207–212 (2017).
Wan, L.-Q. et al. Nonenzymatic stereoselective S-glycosylation of polypeptides and proteins. J. Am. Chem. Soc. 143, 11919–11926 (2021).
Zhang, L. et al. Visible-light-mediated synthesis of non-anomeric S-aryl glycosides via a photoactive electron-donor–acceptor complex. Chem. Commun. 59, 13759–13762 (2023).
Andrews, S., Becker, J. J. & Gagné, M. R. Intermolecular addition of glycosyl halides to alkenes mediated by visible light. Angew. Chem. Int. Ed. Engl. 49, 7274–7276 (2010).
Andrews, S., Becker, J. J. & Gagné, M. R. A photoflow reactor for the continuous photoredox-mediated synthesis of C-glycoamino acids and C-glycolipids. Angew. Chem. Int. Ed. Engl. 51, 4140–4143 (2012).
Wang, Q. et al. Iron-catalysed reductive cross-coupling of glycosyl radicals for the stereoselective synthesis of C-glycosides. Nat. Synth. 1, 235–244 (2022).
Wei, Y., Ben-zvi, B. & Diao, T. Diastereoselective synthesis of aryl C-glycosides from glycosyl esters via C−O bond homolysis. Angew. Chem. Int. Ed. Engl. 60, 9433–9438 (2021).
Zhu, F. et al. Catalytic and photochemical strategies to stabilized radicals based on anomeric nucleophiles. J. Am. Chem. Soc. 142, 11102–11113 (2020).
Liu, J. & Gong, H. Stereoselective preparation of α-C-vinyl/aryl glycosides via nickel-catalyzed reductive coupling of glycosyl halides with vinyl and aryl halides. Org. Lett. 20, 7991–7995 (2018).
Zhang, C. et al. Direct synthesis of unprotected aryl C-glycosides by photoredox Ni-catalysed cross-coupling. Nat. Synth. 2, 251–260 (2023).
Zhang, C. et al. Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation. Nat. Chem. 14, 686–694 (2022).
Zuo, H., Zhang, C., Zhang, Y. & Niu, D. Base-promoted glycosylation allows protecting group-free and stereoselective O-glycosylation of carboxylic acids. Angew. Chem. Int. Ed. Engl. 62, e202309887 (2023).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Alvarez, E. M. et al. O-, N- and C-bicyclopentylation using thianthrenium reagents. Nat. Synth. 2, 548–556 (2023).
Chen, J. J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Bissember, A. C., Lundgren, R. J., Creutz, S. E., Peters, J. C. & Fu, G. C. Transition-metal-catalyzed alkylations of amines with alkyl halides: photoinduced, copper-catalyzed couplings of carbazoles. Angew. Chem. Int. Ed. Engl. 52, 5129–5133 (2013).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C-N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Caiger, L., Zhao, H., Constantin, T., Douglas, J. J. & Leonori, D. The merger of aryl radical-mediated halogen-atom transfer (XAT) and copper catalysis for the modular cross-coupling-type functionalization of alkyl iodides. ACS Catal. 13, 4985–4991 (2023).
Dong, X.-Y., Li, Z.-L., Gu, Q.-S. & Liu, X.-Y. Ligand development for copper-catalyzed enantioconvergent radical cross-coupling of racemic alkyl halides. J. Am. Chem. Soc. 144, 17319–17329 (2022).
Górski, B., Barthelemy, A.-L., Douglas, J. J., Juliá, F. & Leonori, D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 4, 623–630 (2021).
Singh, Y., Geringer, S. A. & Demchenko, A. V. Synthesis and glycosidation of anomeric halides: evolution from early studies to modern methods of the 21st Century. Chem. Rev. 122, 11701–11758 (2022).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & Macmillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Oka, N., Mori, A., Suzuki, K. & Ando, K. Stereoselective synthesis of ribofuranoid exo-glycals by one-pot Julia olefination using ribofuranosyl sulfones. J. Org. Chem. 86, 657–673 (2021).
Wang, Q., Lee, B. C., Song, N. & Koh, M. J. Stereoselective C-aryl glycosylation by catalytic cross-coupling of heteroaryl glycosyl sulfones. Angew. Chem. Int. Ed. Engl. 62, e202301081 (2023).
Lowry, M. S. et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 17, 5712–5719 (2005).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Lemaire, C. F. et al. Fast production of highly reactive no-carrier-added [18F]fluoride for the labeling of radiopharmaceuticals. Angew. Chem. Int. Ed. Engl. 49, 3161–3164 (2010).
Miao, W. et al. Iron-catalyzed difluoromethylation of arylzincs with difluoromethyl 2-pyridyl sulfone. J. Am. Chem. Soc. 140, 880–883 (2018).
Trost, B. M. & Kalnmals, C. A. Sulfones as chemical chameleons: versatile synthetic equivalents of small-molecule synthons. Chem. Eur. J. 25, 11193–11213 (2019).
Nambo, M., Maekawa, Y. & Crudden, C. M. Desulfonylative transformations of sulfones by transition-metal catalysis, photocatalysis, and organocatalysis. ACS Catal. 12, 3013–3032 (2022).
Corpas, J., Kim-Lee, S.-H., Mauleón, P., Arrayás, R. G. & Carretero, J. C. Beyond classical sulfone chemistry: metal- and photocatalytic approaches for C–S bond functionalization of sulfones. Chem. Soc. Rev. 51, 6774–6823 (2022).
Zhu, F. & Walczak, M. A. Stereochemistry of transition metal complexes controlled by the metallo-anomeric effect. J. Am. Chem. Soc. 142, 15127–15136 (2020).
Bordwell, F. G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 21, 456–463 (1988).
Lõkov, M. et al. On the basicity of conjugated nitrogen heterocycles in different media. Eur. J. Org. Chem. 2017, 4475–4489 (2017).
Acknowledgements
This work was supported by the National Key R&D Programme of China (no. 2022YFA1504303), the National Natural Science Foundation of China (no. 92256302), Frontiers Science Center for New Organic Matter (no. 63181206), Fundamental Research Funds for the Central Universities (no. NKU63231195), Haihe Laboratory of Sustainable Chemical Transformations (to G.C.) and the Ministry of Education of Singapore Academic Research Fund Tier 2 (no. A-8000941-00-00 to M.J.K.).
Author information
Authors and Affiliations
Contributions
Q.S. was responsible for the initial discovery of the Cu-catalysed N-glycosylation reaction and conducted most of the substrate scope exploration and mechanistic studies. Q.W. was heavily involved in reaction optimization and mechanistic studies. W.Q. helped with expansion of substrate scope. K.J. conducted reduction potential measurement experiments. G.H. supervised parts of the project. M.J.K. supervised the project and edited the paper. G.C. oversaw the entire project and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Feng Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Detailed synthetic procedures, additional control experiments, compound characterization, liquid chromatography–mass spectrometry tracing, X-ray crystallography and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 51 (no. CCDC 2266734).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Q., Wang, Q., Qin, W. et al. N-glycoside synthesis through combined copper- and photoredox-catalysed N-glycosylation of N-nucleophiles. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00496-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00496-7