Abstract
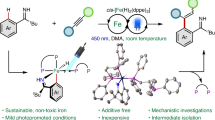
The development of strategies to access boronate esters from ubiquitous aliphatic C−H bonds is of long-standing interest in the synthesis community. Here photoelectrochemically driven C(sp3)−H borylation of alkanes is developed, in which iron, an abundant earth-based resource, is employed as a photoelectrochemical catalyst. Using this protocol, direct borylation of strong alkyl C−H bonds is efficiently achieved at a low oxidation potential of ∼0.3 V and mild conditions. A wide range of structurally diverse alkyl boronic esters, including versatile α-silyl boronic esters, can be accessed with good regioselectivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data relating to the characterization data of materials and products, general methods, optimization studies, experimental procedures, mechanistic studies and NMR spectra are available in the Supplementary Information. All data are also available from the corresponding author upon request.
References
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Fyfe, J. W. B. & Watson, A. J. B. Recent developments in organoboron chemistry: old dogs, new tricks. Chem 3, 31–55 (2017).
Dunfaa, S., Lua, W., Chungua, X. & Chaoa, L. Recent advances in visible-light-promoted transformation of alkyl boron compounds. Chin. J. Org. Chem. 40, 3605–3619 (2020).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C−H activation for the construction of C−B bonds. Chem. Rev. 110, 890–931 (2010).
Ros, A., Fernández, R. & Lassaletta, J. M. Functional group directed C–H borylation. Chem. Soc. Rev. 43, 3229–3243 (2014).
Bisht, R. et al. Metal-catalysed C–H bond activation and borylation. Chem. Soc. Rev. 51, 5042–5100 (2022).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Hu, J., Lv, J. & Shi, Z. Emerging trends in C(sp3)–H borylation. Trends Chem. 4, 685–698 (2022).
Chen, H. & Hartwig, J. F. Catalytic, regiospecific end-functionalization of alkanes: rhenium-catalyzed borylation under photochemical conditions. Angew. Chem. Int. Ed. 38, 3391–3393 (1999).
Chen, H., Schlecht, S., Semple, T. C. & Hartwig, J. F. Thermal, catalytic, regiospecific functionalization of alkanes. Science 287, 1995–1997 (2000).
Lawrence, J. D., Takahashi, M., Bae, C. & Hartwig, J. F. Regiospecific functionalization of methyl C−H bonds of alkyl groups in reagents with heteroatom functionality. J. Am. Chem. Soc. 126, 15334–15335 (2004).
Hartwig, J. F. et al. Rhodium boryl complexes in the catalytic, terminal functionalization of alkanes. J. Am. Chem. Soc. 127, 2538–2552 (2005).
Murphy, J. M., Lawrence, J. D., Kawamura, K., Incarvito, C. & Hartwig, J. F. Ruthenium-catalyzed regiospecific borylation of methyl C−H bonds. J. Am. Chem. Soc. 128, 13684–13685 (2006).
Jones, M. R., Fast, C. D. & Schley, N. D. Iridium-Catalyzed sp3 C–H borylation in hydrocarbon solvent enabled by 2,2′-dipyridylarylmethane ligands. J. Am. Chem. Soc. 142, 6488–6492 (2020).
Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 368, 736–741 (2020).
Cook, A. K., Schimler, S. D., Matzger, A. J. & Sanford, M. S. Catalyst-controlled selectivity in the C–H borylation of methane and ethane. Science 351, 1421–1424 (2016).
Smith, K. T. et al. Catalytic borylation of methane. Science 351, 1424–1427 (2016).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Horn, E. J., Rosen, B. R. & Baran, P. S. Synthetic organic electrochemistry: an enabling and innately sustainable method. J. Am. Chem. Soc. Cent. Sci. 2, 302–308 (2016).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Liu, J., Lu, L., Wood, D. & Lin, S. New redox strategies in organic synthesis by means of electrochemistry and photochemistry. J. Am. Chem. Soc. Cent. Sci. 6, 1317–1340 (2020).
Ma, C. et al. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 66, 2412–2429 (2021).
Novaes, L. F. T. et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 50, 7941–8002 (2021).
Zhu, C., Ang, N. W. J., Meyer, T. H., Qiu, Y. & Ackermann, L. Organic electrochemistry: molecular syntheses with potential. J. Am. Chem. Soc. Cent. Sci. 7, 415–431 (2021).
Cheng, X. et al. Recent applications of homogeneous catalysis in electrochemical organic synthesis. CCS Chem. 4, 1120–1152 (2022).
Kawamata, Y. et al. Scalable, electrochemical oxidation of unactivated C–H bonds. J. Am. Chem. Soc. 139, 7448–7451 (2017).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Yu, Y., Guo, P., Zhong, J.-S., Yuan, Y. & Ye, K.-Y. Merging photochemistry with electrochemistry in organic synthesis. Org. Chem. Front. 7, 131–135 (2020).
Huang, H., Steiniger, K. A. & Lambert, T. H. Electrophotocatalysis: combining light and electricity to catalyze reactions. J. Am. Chem. Soc. 144, 12567–12583 (2022).
Wu, S., Kaur, J., Karl, T. A., Tian, X. & Barham, J. P. Synthetic molecular photoelectrochemistry: new frontiers in synthetic applications, mechanistic insights and scalability. Angew. Chem. Int. Ed. 61, e202107811 (2022).
Xu, P., Chen, P.-Y. & Xu, H.-C. Scalable photoelectrochemical dehydrogenative cross-coupling of heteroarenes with aliphatic C−H bonds. Angew. Chem. Int. Ed. 59, 14275–14280 (2020).
Huang, H., Strater, Z. M. & Lambert, T. H. Electrophotocatalytic C–H functionalization of ethers with high regioselectivity. J. Am. Chem. Soc. 142, 1698–1703 (2020).
Shen, T. & Lambert, T. H. Electrophotocatalytic diamination of vicinal C–H bonds. Science 371, 620–626 (2021).
Shen, T. & Lambert, T. H. C–H amination via electrophotocatalytic Ritter-type reaction. J. Am. Chem. Soc. 143, 8597–8602 (2021).
Shen, T., Li, Y.-L., Ye, K.-Y. & Lambert, T. H. Electrophotocatalytic oxygenation of multiple adjacent C–H bonds. Nature 614, 275–280 (2023).
Niu, L. et al. Manganese-catalyzed oxidative azidation of C(sp3)–H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 142, 17693–17702 (2020).
Wang, F. & Stahl, S. S. Merging photochemistry with electrochemistry: functional-group tolerant electrochemical amination of C(sp3)−H Bonds. Angew. Chem. Int. Ed. 58, 6385–6390 (2019).
Capaldo, L., Quadri, L. L., Merli, D. & Ravelli, D. Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C–H bonds. Chem. Commun. 57, 4424–4427 (2021).
Liu, Y. et al. Time-resolved EPR revealed the formation, structure, and reactivity of N-centered radicals in an electrochemical C(sp3)–H arylation reaction. J. Am. Chem. Soc. 143, 20863–20872 (2021).
Cai, C.-Y. et al. Photoelectrochemical asymmetric catalysis enables site- and enantioselective cyanation of benzylic C–H bonds. Nat. Catal. 5, 943–951 (2022).
Fan, W. et al. Electrophotocatalytic decoupled radical relay enables highly efficient and enantioselective benzylic C–H functionalization. J. Am. Chem. Soc. 144, 21674–21682 (2022).
Tan, Z., He, X., Xu, K. & Zeng, C. Electrophotocatalytic C−H functionalization of N-heteroarenes with unactivated alkanes under external oxidant-free conditions. ChemSusChem 15, e202102360 (2022).
Zou, L., Wang, X., Xiang, S., Zheng, W. & Lu, Q. Paired oxidative and reductive catalysis: breaking the potential barrier of electrochemical C(sp3)−H alkenylation. Angew. Chem. Int. Ed. 62, e202301026 (2023).
Corcé, V., Ollivier, C. & Fensterbank, L. Boron, silicon, nitrogen and sulfur-based contemporary precursors for the generation of alkyl radicals by single electron transfer and their synthetic utilization. Chem. Soc. Rev. 51, 1470–1510 (2022).
Juliá, F. Ligand-to-metal charge transfer (LMCT) photochemistry at 3d-metal complexes: an emerging tool for sustainable organic synthesis. ChemCatChem 14, e202200916 (2022).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem. Int. Ed. 60, 21100–21115 (2021).
Chowdhury, R., Elek, G. Z., Meana-Baamonde, B. & Mendoza, A. Modular synthesis of (borylmethyl)silanes through orthogonal functionalization of a carbon atom. Org. Lett. 25, 1935–1940 (2023).
Ohmura, T., Sasaki, I., Torigoe, T. & Suginome, M. A (borylmethyl)silane bearing three hydrolyzable groups on silicon: synthesis via iridium-catalyzed C(sp3)–H borylation and conversion to functionalized siloxanes. Organometallics 35, 1601–1603 (2016).
Ohmura, T., Torigoe, T. & Suginome, M. Functionalization of tetraorganosilanes and permethyloligosilanes at a methyl group on silicon via iridium-catalyzed C(sp3)–H borylation. Organometallics 32, 6170–6173 (2013).
Ohmura, T., Torigoe, T. & Suginome, M. Catalytic functionalization of methyl group on silicon: iridium-catalyzed C(sp3)–H Borylation of Methylchlorosilanes. J. Am. Chem. Soc. 134, 17416–17419 (2012).
Huang, B., Sun, Z. & Sun, G. Recent progress in cathodic reduction-enabled organic electrosynthesis: trends, challenges, and opportunities. eScience 2, 243–277 (2022).
Bonet, A., Pubill-Ulldemolins, C., Bo, C., Gulyás, H. & Fernández, E. Transition-metal-free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 50, 7158–7161 (2011).
Fu, N., Sauer, G. S. & Lin, S. Electrocatalytic radical dichlorination of alkenes with nucleophilic chlorine sources. J. Am. Chem. Soc. 139, 15548–15553 (2017).
Sang, R. et al. Copper-mediated dehydrogenative C(sp3)–H borylation of alkanes. J. Am. Chem. Soc. 145, 15207–15217 (2023).
Sowndarya, S. V. S., St, John, P. C. & Paton, R. S. A quantitative metric for organic radical stability and persistence using thermodynamic and kinetic features. Chem. Sci. 12, 13158–13166 (2021).
Manley, D. W. & Walton, J. C. A clean and selective radical homocoupling employing carboxylic acids with titania photoredox catalysis. Org. Lett. 16, 5394–5397 (2014).
Tu, J.-L., Hu, A.-M., Guo, L. & Xia, W. Iron-catalyzed C(sp3)–H borylation, thiolation, and sulfinylation enabled by photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 145, 7600–7611 (2023).
Liu, W. et al. Single-bond cleavage of alcohols. Org. Lett. 23, 8413–8418 (2021).
Dai, Z.-Y., Zhang, S.-Q., Hong, X., Wang, P.-S. & Gong, L.-Z. A practical FeCl3/HCl photocatalyst for versatile aliphatic C–H functionalization. Chem Catal. 2, 1211–1222 (2022).
Wang, B. et al. Electrochemical borylation of alkyl halides: fast, scalable access to alkyl boronic esters. J. Am. Chem. Soc. 143, 12985–12991 (2021).
Zou, L., Xiang, S., Sun, R. & Lu, Q. Selective C(sp3)–H arylation/alkylation of alkanes enabled by paired electrocatalysis. Nat. Commun. 14, 7992 (2023).
Zhong, P.-F. et al. Photoelectrochemical oxidative C(sp3)−H borylation of unactivated hydrocarbons. Nat. Commun. 14, 6530 (2023).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant no. 22271227 for Q.L.) and Wuhan University.
Author information
Authors and Affiliations
Contributions
Q.L. conceived and directed the project. Y.C. conducted most of the experimental studies. C.H. supported performance of synthetic experiments. Q.L. wrote the paper. All authors discussed the results, analysed the data and prepared the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Hai-Chao Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary discussion, Figs. 1–30 and Tables 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Y., Huang, C. & Lu, Q. Photoelectrochemically driven iron-catalysed C(sp3)−H borylation of alkanes. Nat. Synth 3, 537–544 (2024). https://doi.org/10.1038/s44160-023-00480-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00480-7