Abstract
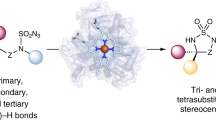
The selective conversion of mixtures of Z/E alkenes into chiral products is a synthetic challenge. Biocatalytic strategies can transform isomeric alkenes into stereopure compounds, but enzymes typically convert only one alkene isomer, limiting the overall yield. Additional strategies have been used to interconvert alkene isomers, often at the cost of increasing energy consumption and chemical waste. Here we present engineered haemoproteins derived from a bacterial cytochrome P450 that efficiently catalyse α-carbonyl alkylation of isomeric silyl enol ethers, producing stereopure products. Through screening and directed evolution, we generated P450BM3 variant P411-SCA-5188, which catalyses stereoconvergent carbene transfer in Escherichia coli with high efficiency and stereoselectivity to various Z/E mixtures of silyl enol ethers. In contrast to established stereospecific transformations that leave one isomer unreacted, P411-SCA-5188 converts both isomers to a stereopure product. This biocatalytic approach simplifies the synthesis of chiral α-branched ketones by eliminating the need for stoichiometric chiral auxiliaries, strongly basic alkali-metal enolates and harsh conditions, delivering products with high efficiency and excellent chemo- and stereoselectivities.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The original materials and data that support the findings of this study are available within the paper and its Supplementary Information.
References
Straathof, A. J., Panke, S. & Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 13, 548–556 (2002).
Schoemaker, H. E., Mink, D. & Wubbolts, M. G. Dispelling the myths—biocatalysis in industrial synthesis. Science 299, 1694–1697 (2003).
Nestl, B. M., Nebel, B. A. & Hauer, B. Recent progress in industrial biocatalysis. Curr. Opin. Chem. Biol. 15, 187–193 (2011).
Wu, S., Snajdrova, R., Moore, J. C., Baldenius, K. & Bornscheuer, U. T. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 60, 88–119 (2021).
Bell, E. L. et al. Biocatalysis. Nat. Rev. Methods Primers 1, 46 (2021).
Patel, R. N. Biocatalysis for synthesis of pharmaceuticals. Bioorg. Med. Chem. 26, 1252–1274 (2018).
Wu, S., Zhou, Y. & Li, Z. Biocatalytic selective functionalisation of alkenes via single-step and onepot multi-step reactions. Chem. Commun. 55, 883–896 (2019).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: sereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Beller, M., Seayad, J., Tillack, A. & Jiao, H. Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: recent developments and trends. Angew. Chem. Int. Ed. 43, 3368–3398 (2004).
Rodriguez-Ruiz, V. et al. Recent developments in alkene hydro-functionalisation promoted by homogeneous catalysts based on earth abundant elements: formation of C–N, C–O and C–P bond. Dalton Trans. 44, 12029–12059 (2015).
Coombs, J. R. & Morken, J. P. Catalytic enantioselective functionalization of unactivated terminal alkenes. Angew. Chem. Int. Ed. 55, 2636–2649 (2016).
Yanto, Y. et al. Asymmetric bioreduction of alkenes using ene-teductases YersER and KYE1 and effects of organic solvents. Org. Lett. 13, 2540–2543 (2011).
Yang, L.-C., Deng, H. & Renata, H. Recent progress and developments in chemoenzymatic and biocatalytic dynamic kinetic resolution. Org. Process Res. Dev. 26, 1925–1943 (2022).
Demming, R. M. et al. Asymmetric enzymatic hydration of unactivated, aliphatic alkenes. Angew. Chem. Int. Ed. 58, 173–177 (2019).
Stueckler, C. et al. Stereocomplementary bioreduction of α,β-unsaturated dicarboxylic acids and dimethyl esters using enoate reductases: enzyme- and substrate-based stereocontrol. Org. Lett. 9, 5409–5411 (2007).
Wu, S. et al. Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase. ACS Catal. 4, 409–420 (2014).
Williams, J. M. J. Preparation of Alkenes: A Practical Approach (Oxford Univ. Press, 1996).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E→Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Litman, Z. C., Wang, Y., Zhao, H. & Hartwig, J. F. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis. Nature 560, 355–359 (2018).
Mohr, J. T., Moore, J. T. & Stoltz, B. M. Enantioconvergent catalysis. Beilstein J. Org. Chem. 12, 2038–2045 (2016).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Kroutil, W., Mischitz, M. & Faber, K. Deracemization of (±)-2,3-disubstituted oxiranes via biocatalytic hydrolysis using bacterial epoxide hydrolases: kinetics of an enantioconvergent process. J. Chem. Soc., Perkin Trans. 1, 3629–3636 (1997).
Chiappe, C. et al. Biocatalysis in ionic liquids: the stereoconvergent hydrolysis of trans-β-methylstyrene oxide catalyzed by soluble epoxide hydrolase. J. Mol. Catal. B: Enzym. 27, 243–248 (2004).
Yang, Y., Cho, I., Qi, X., Liu, P. & Arnold, F. H. An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds. Nat. Chem. 11, 987–993 (2019).
Mao, R. et al. Enantio- and diastereoenriched enzymatic synthesis of 1,2,3-polysubstituted cyclopropanes from (Z/E)-trisubstituted enol acetates. J. Am. Chem. Soc. 145, 16176–16185 (2023).
Carreira, E. M. & Kvaerno, L. in Classics in Stereoselective Synthesis, Chapter 3: α-Functionalization of Enolates, 69–102 (Wiley-VCH, 2009).
Dugger, R. W., Ragan, J. A. & Ripin, D. H. B. Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org. Process Res. Dev. 9, 253–258 (2005).
Wright, T. B. & Evans, P. A. Catalytic enantioselective alkylation of prochiral enolates. Chem. Rev. 121, 9196–9242 (2021).
Evans, D. A., Ennis, M. D. & Mathre, D. J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 104, 1737–1739 (1982).
Myers, A. G., Yang, B. H., Chen, H. & Gleason, J. L. Use of pseudoephedrine as a practical chiral auxiliary for asymmetric synthesis. J. Am. Chem. Soc. 116, 9361–9362 (1994).
Sruthi, P. R. & Anas, S. An overview of synthetic modification of nitrile group in polymers and applications. J. Polym. Sci. 58, 1039–1061 (2020).
Wang, X. et al. Nitrile-containing pharmaceuticals: target, mechanism of action, and their SAR studies. RSC Med. Chem. 12, 1650–1671 (2021).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Prier, C. K., Zhang, R. K., Buller, A. R., Brinkmann-Chen, S. & Arnold, F. H. Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 9, 629–634 (2017).
Acknowledgements
Support by the National Science Foundation Division of Molecular and Cellular Biosciences (MCB-2016137 to F.H.A.) is gratefully acknowledged. R.M. acknowledges support from the Swiss National Science Foundation (SNSF) Early Mobility Postdoctoral Fellowship (P2ELP2_195118). D.J.W. acknowledges support from the National Science Foundation Graduate Research Fellowship (DGE-1745301). K.M.S. acknowledges support from NIH Ruth L. Kirschstein National Research Service Award (1F32GM145123-01A1). Support by the National Science Foundation Division of Chemistry (CHE-2153972 to K.N.H.) and the Alexander von Humboldt Foundation (Feodor Lynen Fellowship, T.R.) is gratefully acknowledged. We thank S. C. Virgil for the maintenance of the Caltech Center for Catalysis and Chemical Synthesis (3CS). We thank M. Shahgoli for mass spectrometry assistance. We thank D. VanderVelde for the maintenance of the Caltech NMR facility. We also thank S. Brinkmann-Chen for the helpful discussions and comments on the manuscript. Calculations were performed on the Hoffman2 cluster at the University of California, Los Angeles.
Author information
Authors and Affiliations
Contributions
R.M. conceptualized and designed the overall project under the guidance of F.H.A. R.M. and D.J.W. conducted the initial screening of haem proteins. D.M.T. performed the directed evolution experiments, with support from S.J.W. R.M. and D.J.W. investigated the substrate scope and reaction mechanism. K.M.S. purified and obtained both (Z)-1a and (E)-1a. T.R. carried out the computational studies with K.N.H. providing guidance. R.M. and F.H.A. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Source Data Fig. 2
Source data for Fig. 2b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, R., Taylor, D.M., Wackelin, D.J. et al. Biocatalytic, stereoconvergent alkylation of (Z/E)-trisubstituted silyl enol ethers. Nat. Synth 3, 256–264 (2024). https://doi.org/10.1038/s44160-023-00431-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00431-2