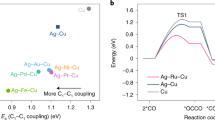
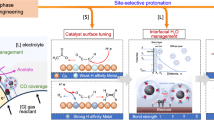
Abstract
Acetic acid is an important chemical feedstock. The electrocatalytic synthesis of acetic acid from CO2 offers a low-carbon alternative to traditional synthetic routes, but the direct reduction from CO2 comes with a CO2 crossover energy penalty. CO electroreduction bypasses this, which motivates the interest in a cascade synthesis approach of CO2 to CO followed by CO to acetic acid. Here we report a catalyst design strategy in which off-target intermediates (such as ethylene and ethanol) in the reduction of CO to acetate are destabilized. On the optimized Ag–CuO2 catalyst, this destabilization of off-target intermediates leads to an acetate Faradaic efficiency of 70% at 200 mA cm−2. We demonstrate 18 hours of stable operation in a membrane electrode assembly; the system produced 5 wt% acetate at 100 mA cm−2 and a full-cell energy efficiency of 25%, a twofold improvement on the highest energy-efficient electrosynthesis in prior reports.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. All the other relevant data supporting the findings of this study, which include techno-economic assessment methodologies, computational details and other experimental, microscopic and spectroscopic analyses, are available within the article and its Supplementary Information.
References
Schiffer, Z. J. & Manthiram, K. Electrification and decarbonization of the chemical industry. Joule 1, 10–14 (2017).
Luderer, G. et al. Impact of declining renewable energy costs on electrification in low-emission scenarios. Nat. Energy 7, 32–42 (2022).
Tackett, B. M., Gomez, E. & Chen, J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2, 381–386 (2019).
Le Berre, C., Serp, P., Kalck, P. & Torrence, G. P. in Ullmann’s Encyclopedia of Industrial Chemistry (2014); https://doi.org/10.1002/14356007.A01_045.PUB3
Medrano-García, J. D., Ruiz-Femenia, R. & Caballero, J. A. Revisiting classic acetic acid synthesis: optimal hydrogen consumption and carbon dioxide utilization. Comput. Aided Chem. Eng. 46, 145–150 (2019).
Alerte, T. et al. Downstream of the CO2 electrolyzer: assessing the energy intensity of product separation. ACS Energy Lett. 6, 4405–4412 (2021).
Mardle, P., Cassegrain, S., Habibzadeh, F., Shi, Z. & Holdcroft, S. Carbonate ion crossover in zero-gap, KOH anolyte CO2 electrolysis. J. Phys. Chem. C 125, 25446–25454 (2021).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Zhang, X., Song, Y., Wang, G. & Bao, X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: recent advance in cathodes. J. Energy Chem. 26, 839–853 (2017).
Ramdin, M. et al. Electroreduction of CO2/CO to C2 products: process modeling, downstream separation, system integration, and economic analysis. Ind. Eng. Chem. Res. 60, 17862–17880 (2021).
Wang, L. et al. Power-to-fuels via solid-oxide electrolyzer: operating window and techno-economics. Renew. Sustain. Energy Rev. 110, 174–187 (2019).
Gurudayal, G. et al. Sequential cascade electrocatalytic conversion of carbon dioxide to C–C coupled products. ACS Appl. Energy Mater. 2, 4551–4559 (2019).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Romero Cuellar, N. S. et al. Two-step electrochemical reduction of CO2 towards multi-carbon products at high current densities. J. CO2 Util. 36, 263–275 (2020).
Zhu, P. et al. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. Proc. Natl Acad. Sci. USA 118, e2010868118 (2021).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23 (2005).
Zhang, Y. J., Sethuraman, V., Michalsky, R. & Peterson, A. A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 4, 3742–3748 (2014).
Herzog, A. et al. Operando investigation of Ag-decorated Cu2O nanocube catalysts with enhanced CO2 electroreduction toward liquid products. Angew. Chem. Int. Ed. 60, 7426–7435 (2021).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Lee, S. H. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
Verdaguer-Casadevall, A. et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 137, 9808–9811 (2015).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Wang, L. et al. Electrochemically converting carbon monoxide to liquid fuels by directing selectivity with electrode surface area. Nat. Catal. 2, 702–708 (2019).
Santatiwongchai, J., Faungnawakij, K. & Hirunsit, P. Comprehensive mechanism of CO2 electroreduction toward ethylene and ethanol: the solvent effect from explicit water–Cu(100) interface models. ACS Catal. 11, 9688–9701 (2021).
Calle-Vallejo, F. & Koper, M. T. M. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 52, 7282–7285 (2013).
Wang, L. et al. Electrochemical carbon monoxide reduction on polycrystalline copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 8, 7445–7454 (2018).
Garza, A. J., Bell, A. T. & Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 8, 1490–1499 (2018).
Chernyshova, I. V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Gunathunge, C. M., Ovalle, V. J., Li, Y., Janik, M. J. & Waegele, M. M. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 8, 7507–7516 (2018).
Oda, I., Ogasawara, H. & Ito, M. Carbon monoxide adsorption on copper and silver electrodes during carbon dioxide electroreduction studied by infrared reflection absorption spectroscopy and surface-enhanced Raman spectroscopy. Langmuir 12, 1094–1097 (1996).
Gao, J. et al. Selective C–C coupling in carbon dioxide electroreduction via efficient spillover of intermediates as supported by operando Raman spectroscopy. J. Am. Chem. Soc. 141, 18704–18714 (2019).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Shan, W. et al. In situ surface-enhanced Raman spectroscopic evidence on the origin of selectivity in CO2 electrocatalytic reduction. ACS Nano 14, 11363–11372 (2020).
Fielicke, A., Gruene, P., Meijer, G. & Rayner, D. M. The adsorption of CO on transition metal clusters: a case study of cluster surface chemistry. Surf. Sci. 603, 1427–1433 (2009).
Sandberg, R. B., Montoya, J. H., Chan, K. & Nørskov, J. K. CO–CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Quilès, F. & Burneau, A. Infrared and Raman spectra of alkaline-earth and copper(II) acetates in aqueous solutions. Vib. Spectrosc. 16, 105–117 (1998).
Bohra, D. et al. Lateral adsorbate interactions inhibit HCOO− while promoting CO selectivity for CO2 electrocatalysis on silver. Angew. Chem. 131, 1359–1363 (2019).
Shao, F. et al. In situ spectroelectrochemical probing of CO redox landscape on copper single-crystal surfaces. Proc. Natl Acad. Sci. USA 119, e2118166119 (2022).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2019).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Yang, P. P. et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels. J. Am. Chem. Soc. 142, 6400–6408 (2020).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Martić, N. et al. Ag2Cu2O3–a catalyst template material for selective electroreduction of CO to C2+. products. Energy Environ. Sci. 13, 2993–3006 (2020).
Acknowledgements
We acknowledge the support of this work by the Ontario Research Foundation—Research Excellence Program (no. ORF-RE08-034, E.H.S.), the Natural Sciences and Engineering Research Council (NSERC) of Canada (no. RGPIN-2017-06477, E.H.S.) and Suncor Canada. I.G. acknowledges the European Union’s Horizon 2020 research and innovation programme under a Marie Sklodowska-Curie grant (agreement no. 846107). DFT calculations were performed on the Niagara supercomputer at the SciNet HPC Consortium. We acknowledge the computational resources supported by SciNet, which is funded by the University of Toronto, the Ontario Research Fund—Research Excellence Program, the Government of Ontario and the Canada Foundation for Innovation. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory and was supported by the US DOE under contract no. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners. This research used resources of the European Synchrotron Radiation Facility at beamline ID26 during the experimental session MA5352 (https://doi.org/10.15151/ESRF-ES-744180074). We thank D. Motta Meira from the 20BM beamline for assistance in collecting the XAS data. We thank R. Wolowiec and D. Kopilovic for their kind technical assistance, S. Boccia from the Ontario Centre for the Characterization of Advanced Materials (OCCAM) of the University of Toronto for the electron microscopy imaging and A. Ip for general input on the paper. We thank Y.-C. Chu and H. M. Chen in the National Taiwan University for conducting in situ XAS experiments. We thank C.-W. Bao in TPS 44A1, National Synchrotron Radiation Research Center, Taiwan, for help with tuning the incident beam of the XAS. We acknowledge support from the Ministry of Science and Technology, Taiwan (contract nos. MOST 110-2628-M-002-001-RSP and 110-2113-M-153-001).
Author information
Authors and Affiliations
Contributions
E.H.S. supervised the project. R.D., I.G. and B.-H.L. conceived the idea. R.D., B.-H.L. and I.G. designed and performed the experiments. R.D., with the help of P.O., carried out the DFT calculations. J.A. performed the XAS experiments. J.A., I.G. and R.D. analysed the XAS data. A.S.R. performed the XPS experiments. A.S.R. and I.G. analysed the XPS data. M.P., B.-H.L. and R.D. performed the TEM and SEM measurements. R.K.M., E.S., J.W., S.P., G.L. and J.Z. contributed to the data interpretation, material synthesis and characterization. R.K.M., C.O. and D.S. assisted with the electrochemical system design. R.D., I.G., B.-H.L., P.O. and E.H.S. wrote the manuscript. All the authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Dehui Deng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes 1–4, Figs. 1–38 and Tables 1–11.
Source data
Source Data Fig. 2
Physical characterization data.
Source Data Fig. 3
Electrochemical testing data.
Source Data Fig. 4
Computational calculations.
Source Data Fig. 5
Operando and post catalysis characterization data.
Source Data Fig. 6
Electrochemical testing data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dorakhan, R., Grigioni, I., Lee, BH. et al. A silver–copper oxide catalyst for acetate electrosynthesis from carbon monoxide. Nat. Synth 2, 448–457 (2023). https://doi.org/10.1038/s44160-023-00259-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00259-w
This article is cited by
-
High yield electrosynthesis of oxygenates from CO using a relay Cu-Ag co-catalyst system
Nature Communications (2024)
-
Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity
Nature Communications (2024)