Abstract
Arctic climate change is leading to sea-ice attrition in the Last Ice Area along the northern coast of Canada and Greenland, but less attention has been given to the associated land-based ecosystems. Here we evaluated bacterial community structure in a hydrologically coupled cryo-ecosystem in the region: Thores Glacier, proglacial Thores Lake, and its outlet to the sea. Deep amplicon sequencing revealed that Polaromonas was ubiquitous, but differed genetically among diverse niches. Surface glacier-ice was dominated by Cyanobacteria, while the perennially ice-capped, well-mixed water column of Thores Lake had a unique assemblage of Chloroflexi, Actinobacteriota, and Planctomycetota. Species richness increased downstream, but glacier microbes were little detected in the lake, suggesting strong taxonomic sorting. Ongoing climate change and the retreat of Thores Glacier would lead to complete drainage and loss of the lake microbial ecosystem, indicating the extreme vulnerability of diverse cryohabitats and unique microbiomes in the Last Ice coastal margin.
Similar content being viewed by others
Introduction
The Canadian Arctic Archipelago (CAA), one of the most glaciated areas of the Arctic, has experienced rapid ice attrition over the past decades, especially at the coastal margin of the Last Ice Area (LIA). Wind and currents push the sea ice up against the coast of the far northern islands in the CAA, resulting in some of the thickest sea ice of the Arctic Ocean [1] and persistent cold conditions for habitats on and near the land such as ice shelves, glaciers and perennially ice-capped lakes. The LIA is therefore considered an enduring refuge and conservation site for polar biota, including microbes, in the face of global warming [2]. However, over the last 30 years, ice shelves in the area have contracted by 42%, glaciers have shrunk measurably, and multiyear landfast ice at this margin, some of it 50 years old, has almost completely disappeared [3]. In the coastal lands adjoining the LIA, lakes that were previously capped by thick ice have recently experienced complete ice break-up and loss in summer, causing disruption of these ecosystems by altered stratification and mixing regimes [4]. Climate-driven change in the marine environment therefore has repercussions for adjacent terrestrial cryohabitats, and for freshwater ecosystem stability.
Ice habitats in Arctic marine and terrestrial environments are known to harbor complex microbial communities [5, 6] and rapid microbial evolutionary processes [7]. Degradation of the cryosphere therefore threatens its unique biodiversity and may release ice-dwelling microbes from glaciers [8], permafrost [9] or snow [10] into downstream habitats, resulting in interactions with unknown consequences. Certain taxa such as the proteobacterial genus Polaromonas are known to thrive in these cold environments [11, 12], with some evidence that there may be local adaptation to exploit specific habitats [10, 13]. However, little is known about microbial diversity and connections among ice-dependent habitats in the far North and their sensitivity to change, notably in the terrestrial cryosphere and the associated meltwater habitats. High Arctic freshwaters exhibit the greatest sensitivity to rapid, warming-enduced state shifts [14], and are likely to be transformed dramatically by changes in their adjacent cryosphere.
Here, we evaluated the bacterial diversity of proglacial Thores Lake (Fig. 1), an ice-dammed waterbody that lies 50 km inland from the Arctic Ocean, and 16 km upstream of a fiord in the LIA [15]. Unlike many other lakes in the region, it appears to be buffered by change as evidenced by its adjacent glacier, which is among the more stable of northern Ellesmere Island glaciers [16]. Ice-dammed lakes like Thores interact with their adjoining glaciers, and the ice-contact zone is the site of meltwater exchanges and thermal feedback [17]. Understanding the processes underway in these transition zones of proglacial lakes is critical to predicting the fate of land-terminating glaciers, and can also provide valuable information about the release of ice-dwelling organisms into the hydrosphere.
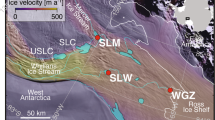
a Location of the Thores system on northern Ellesmere Island, near Disraeli Fiord (inset). b Interface between Thores Glacier and Thores Lake. c Map of sampling sites collected along flow order in Thores watershed and outflow (n = 14). d Samples were collected at each site either from ice (glacier, lake ice) or water (lake, river, meltwater).
We hypothesized that cold-dwelling Polaromonas would be widely distributed across the Thores glacier-lake-outflow continuum, but that the diversity of cryohabitats in this system would favor niche adaptation within the genus. We further surmized that despite this differentiation, Thores Lake and its outflow would show evidence of connectivity to the cryosphere, with a strong imprint of glacier and lake ice microbes on aquatic communities. Such connectivity has been observed in a snow-lake-outflow system [10] on Ward Hunt Island, which has been impacted by climate change at the LIA coast [18]. We addressed these hypotheses by accessing the remote Thores Lake area by helicopter in mid-summer and sampling the glacier surface, the interface zone with the lake, sites throughout the lake, perennial lake ice, and the river outflow at its outlet and 16 km downstream, just before its entry into the fiord. Deep amplicon sequencing of these samples allowed us to identify similarities and differences in bacterial community structure, and the niche separation of Polaromonas phylotypes across this coupled ice-water ecosystem in the LIA terrestrial margin.
Material and methods
Site description
Thores Lake (82.65162°N, -73.663669°W) is an ultraoligotrophic, unstratified proglacial lake [15] located in remote Quttinirpaaq National Park on Ellesmere Island, Nunavut, Canada. The lake has a maximum depth of at least 69 m and an area of 2.4 km2. It is fed by Thores Glacier, which is stable in contrast to many other glaciers on Northern Ellesmere Island [16], and is drained by Thores River into Disraeli Fiord and the Arctic Ocean [15, 16].
Sample collection
Samples were collected in summer (July-August) 2018 and 2019, at the sites shown in Fig. 1. Physico-chemical parameters were measured using an RBRconcerto3 profiling system (RBR Ltd, Ottawa, Canada) that measured temperature, conductivity, chlorophyll-a, dissolved oxygen, and depth. All samples in the study were collected in triplicate. Detailed sampling methods and water chemistry data are presented in Culley et al. [15].
Three 30 cm diameter holes were bored 10 m apart through the ice cover on Thores Lake (115 cm ice thickness). Lake water samples were collected using a 7-liter Limnos sampler at depths of 5, 15, 17, and 40 m, and stored in low-density polyethylene Cubitainers that had been washed with 2% (vol/vol) Contrad 70 liquid detergent (DeconLabs, King of Prussia, PA, USA) and 10% (vol/vol) ACS-grade HCl (Sigma-Aldrich, St. Louis, MO, USA) and rinsed with lake water. Flowing water (rivers, streams, waterfalls) was collected as grab samples into Contrad and HCL-washed Nalgene high density polyethylene (HDPE) bottles. Ice samples were collected with a Mark II corer (Kovacs Enterprise, Roseburg, OR, USA) from lake ice and the glacier, and thawed in sterile 5-liter Whirl-PakTM bags (Whirl-Pak, Madison, WI, USA) in the dark at 4 °C. Snow samples were also collected into opaque HDPE bottles, and thawed in the dark at 4 °C.
Subsamples from water and thawed ice samples were stored at 4 °C in the dark for analysis of total phosphorus (TP), total nitrogen (TN), and dissolved organic carbon (DOC). Subsamples were also filtered onto 25-mm diameter Whatman® GF/F glass-fiber filters (nominal pore size of 0.7 µm) (Sigma-Aldrich) and immediately frozen at −20 °C for pigment analyses. Whole water for sequencing was filtered separately onto Sterivex™ 0.22 µm capsule filters (Millipore, Burlington, MA, USA) in 2018 and onto Anotop® 0.02 µm syringe filters (Whatman, Maidstone, United Kingdom) in 2019. Filters were stored at −20 °C on the field, and at −80 °C at Université Laval until nucleic acid extraction.
Sampling dates and filters used for molecular work are presented in Supplementary Table S1.
Sample processing
TP, TN and DOC samples were analyzed at l’Université du Québec à Montréal (UQAM, Montréal) [19,20,21]. Fatty acids were analyzed by the Fatty Acid Analytical Laboratory (UQAC, Chicoutimi) as described in Wauthy & Rautio [22]. Pigments were analyzed by high-performance liquid chromatography (HPLC), as in Thaler et al. [23]. Water chemistry results for are reported in Supplementary Table S2, and full water chemistry data set and RBR profiles are available online in the Nordicana environmental data archive [24, 25].
Nucleic acids were extracted from Sterivex™ filters with a modified version of the AllPrep DNA/RNA Mini kit (Qiagen, Hilden, Germany), as described in Cruaud et al. [26] and 0.02 µm Anotop® filters were extracted with the MasterPure Complete DNA & RNA purification kit (Epicentre, Madison, WI, USA) using the backflushing technique described by Mueller et al. [27]. Libraries were prepared by 2-step PCR targeting the V4 region of the 16 S rRNA gene (with primers 515 F & 806 R) with the Q5 High-fidelity polymerase (NEB, Ipswich, MA, USA), purified on SparQ Purmag beads (QuantaBio, Beverly, MA, USA) and sequenced on an Illumina MiSeq by paired-end sequencing (2x300bp) at the Institut de biologie intégrative et des systèmes (IBIS, Université Laval). Sequencing yielded a total of 8 845 831 reads (see Supplementary Table S3 for DNA concentration and sequencing yield per sample).
Sequence processing and statistical analyses
Of the 19 samples sequenced, 5 were removed from the subsequent analyses due to low read counts (<14 000 reads, all others between 255 000-380 000 reads; Supplementary Table S3). Reads were processed with DADA2 (v1.14.0) [28] in R (v3.6.1) [29] for quality-filtering, paired-end concatenation, removal of chimeras and identification of exact amplicon sequence variants (ASVs) [30]. This was done using default parameters except in the filterAndTrim() function for truncLen (275,250) and trimLeft (19,20), chosen from the error rate quality graphs. Taxonomy was assigned using SILVA (release 138) [31] and chloroplast-assigned sequences were removed, leaving a total of 6 785 different ASVs across 14 samples. Taxonomy was assigned at least to phylum level for 95.9% of reads. These 14 samples (each containing between 111 707 and 200 611 quality-controlled reads per sample) are presented throughout the manuscript in the order of flow, from upstream habitats to downstream sites (Fig. 1c). For read processing throughout the DADA2 pipeline, see Supplementary Table S3.
ASVs were analyzed in R with the phyloseq{} package (v1.30.0) [32]. A raw ASV table was used for community composition figures, calculating core communities, biomarker analyses, source tracking, and for alpha diversity estimates, while a table transformed by Hellinger standardization with the decostand() function in vegan{} (v2.5-6) [33] was used for beta diversity and ordinations. ASV table manipulations were done using the stringr{} (v1.4.0) [34], DECIPHER{} (v2.14.0) [35], tibble{} (v3.0.2), dplyr{} (v1.0.0) [36] and tidyr{} (v1.1.0) [37] packages in R.
Alpha diversity estimates were calculated with Chao1 and Shannon’s diversity indices with phyloseq{}. Community composition was plotted with ggplot2{} [38] and RColorBrewer{} (v1.1-2) [39], and core microbiome members were identified with the core() function in the microbiome{} package (v1.8.0) [40] (taxa detected in >50% of samples at a relative abundance >1%). Community diversity was calculated using Bray-Curtis’ dissimilarity index and plotted as ordinations in Principal Coordinate Analysis (PCoA) space with phyloseq{}. Analysis of variance comparing habitat type (ice, lake, river) was calculated with the adonis() function, and group dispersion homogeneity was verified with betadisper() in vegan{} (P = 0.153, F = 2.2537, permutations = 999). Photosynthetic oxygen regimes (oxygenic, anoxygenic) were assigned to taxa based on known photosynthesis metabolism in bacteria [41]. Anoxygenic organisms grouped all photosynthetic organisms that did not belong to the Cyanobacteria phylum, and anoxygenic and oxygenic organisms were plotted by relative abundance.
Discriminating features (biomarkers) in ice and water samples were identified with linear discriminant analysis (LDA) effect size (LEfSe) [42] implemented in microbiomeMarker{} (v.0.0.1.9000) [43] (LDA cutoff = 4, Kruskal–Wallis P value cutoff = 0.05, bootstrap = 30). Intersections between Polaromonas in Thores glacier-lake continuum were plotted with UpSetR{} (1.4.0) [44]. The source of ASVs along the flow order was identified by Bayesian approach using SourceTracker [45] (sources = glacier, lake ice, lake water; sinks = lake, lake ice, outflow) (α = 0.001, with mean prediction proportion and standard deviation reported from Gibbs sampling over 10 draws).
The taxonomy of Polaromonas-assigned ASVs was inferred through phylogeny, using partial 16 S sequences from isolates of the representatives of the nine Polaromonas species (P. naphtalenivorans CJ2 [NR_027567.1], P. hydrogenivorans strain DSM 17735 [NR_043540.1], P. glacialis strain Cr4-12 [NR_109013.1], P. cryoconiti strain Cr4-35 [NR_109012.1], P. jejuensis strain JS12-13 [EU030285.1], P. aquatica strain CCUG 29402 [NR_042404.1], P. ginsengisoli strain Gsoil 115 [AB245355.1], P. vacuolata strain 34-P [NR_025958.1], P. eurypsychrophila strain B717-2 [NR_149767.1]). The phylogenetic tree of these species was computed with ClustalOmega [46], rooted with a Rhodoferax (closely related genus [47]) ASV from this study. The tree was plotted and annotated using iTol (v4) [48].
Polaromonas sequences from cryospheric habitats were collected in fasta format from the NCBI Nucleotide database using esearch() and efetch() from the E-utilities package (NCBI) by using the query “txid52972[Organism]” in combination with either of the following keywords: “ice”, “glacier”, “Arctic” and “Antarctica”. This produced a set of 855 unique Polaromonas sequences. From these sequences, whole genomes as well as plasmids were removed, and we retained only sequences from the 16 S rRNA gene (376 sequences). To these we added the 16 S sequences from uncultured bacterium reported to be Polaromonas identified by Darcy et al. [11] (55 sequences) as well as the 12 ASVs from Northern Ellesmere identified as Polaromonas in our pipeline. These sequences were aligned with mafft (v7.453) [49], the alignment was trimmed to the consensus region of 286 bp with MegaX (v10.1.8) [50], and non-aligning sequences were removed with SeaView (v5.0.4) [51], producing a final data set of 363 Polaromonas 16 S genes or gene fragments. The phylogenetic tree was produced as described in the previous paragraph. For accession numbers and references of sequences obtained on NCBI, see Supplementary Table S4.
Results
Polaromonas ubiquity and niche separation
The core microbiome across the Thores glacier-lake-outflow system contained 141 amplicon sequence variants (ASVs), with around one tenth belonging to the Comamonadaceae (4 Polaromonas ASVs, 4 Rhodoferax, 1 Limnohabitans, 1 Delftia, 1 Roseateles and 3 unidentified Comamonadaceae; Supplementary Table S5). This family represented a substantial proportion of all reads from the Thores watershed (between 9 and 59% of reads per sample, average ± SD of 19 ± 14%). Polaromonas were significantly more characteristic of ice than water samples (LDA score = 5.10, P = 0.004, Supplementary Table S6 & Fig. 2a). This genus was detected throughout the glacier-lake-outlet ecosystem (Fig. 2a), outnumbering all other members of the Comamonadaceae (except Rhodoferax at the junction between the outlet and Arctic Ocean in Disraeli Fiord), and represented 19% of reads in the cryosphere samples and 8.2% of lake sequences.
a Relative abundance of main genera of the Comamonadaceae family, including Polaromonas. b Proportion of shared Polaromonas in the glacier-lake continuum between interface, lake ice, glacier, and lake water habitats (with the number of ASVs in each habitat in parentheses). Most Polaromonas phylotypes were shared across all four compartments, while the glacier and lake had 2 unique phylotypes each. Lake and lake ice shared one phylotype. c Phylogenetic tree of Thores Polaromonas ASVs and partial 16 S sequences of previously isolated representatives of the nine Polaromonas species (P. eurypsychrophila NR 149767.1, P. vacuolata NR 025958.1, P. ginsengisoli AB245355.1, P. aquatica NR 042404.1, P. jejuensis EU0302285.1, P hydrogenivorans NR 043540.1, P. cryoconiti NR 109012.1, P. glacialis NR 109013.1, P. naphthalenivorans NR 027567.1). The tree is rooted with a Rhodoferax ASV from the Thores dataset. d The relative abundance of each Thores Polaromonas phylotype is shown by the size of the circle, which is organized by sample type (order and color) is represented by sample type. e Fraction of samples in which each Thores Polaromonas was detected. The waterfall flowing from another glacier downstream of the lake was omitted from this analysis (a total of 13 samples).
While most Polaromonas ASVs were common to all compartments of the glacier-lake continuum (Fig. 2b), there was inter-habitat diversity within the genus (Fig. 2c, d). One Polaromonas ASV (pol1) dominated other members of the genus, accounting for over 25% of reads per sample (Fig. 2c, d). This phylotype, which was closely related to P. ginsengisoli (Fig. 2c) was ubiquitous throughout the continuum (Fig. 2e). Another Polaromonas phylotype (pol2), closely related to P. cryoconiti, was also detected in all compartments, but at much lower relative abundances (5.5% of ASVs in the junction with Disraeli Fiord, maximum of 0.5% of reads in all other samples) (Fig. 2e). Two phylotypes (pol3 & pol5) related to P. eurypsychrophila were most abundant in the lake’s water column, although detected in lower abundances in other compartments, similarly to pol6, related to P. aquatica (Fig. 2d). Two low abundance phylotypes (pol9 & pol10), related to P. aquatica and P. naphthalenivorens (Fig. 2d), were unique to the glacier (Fig. 2b). All phylotypes identified in lake ice were shared with the water column (Fig. 2b), and the interface had no unique Polaromonas phylotypes – all representatives identified in this compartment were shared with the glacier, water column and lake ice (Fig. 2b).
A meta-analysis including sequences from six continents and multiple habitat types showed that the most prevalent Polaromonas phylotype in our analyses (pol1), i.e. the ASV present in the greatest fraction of samples and at the greatest abundance (Fig. 2d, e), was most closely related to isolates from Collins Glacier (Antarctica) and Arikaree Glacier (Colorado) (Fig. 3). Thores Polaromonas phylotypes did not group together, and lake phylotypes (pol3 & pol5) grouped closer to the root of the tree (Fig. 3). The pol12 phylotype (which was related to P. glacialis in the reference tree (Fig. 2c)) grouped with North American glaciers and lakes.
Phylogeny of Polaromonas-identified ASVs from the Thores system, aligned along the V4 region of the 16 S rRNA gene of Polaromonas isolates from Ward Hunt Island (NEI) and around the globe accessed through NCBI. For accession numbers and metadata for isolates, see Supplementary Table S4.
Cryohabitat diversity and bacterial assemblages
The Thores lake-glacier continuum encompassed different types of cryospheric habitats, including glacier ice, glacier-associated habitats such as cryoconite holes, and lake ice. While Polaromonas dominated, they were only part of the rich assemblages identified in these cryohabitats. The Deinococcota phylum (including Deinococcus) (LDA score = 4.87, P = 0.002), Bacteroidota (including Cytophagales) (LDA score = 5.00, P = 0.004), Pseudanabaenales (LDA score = 4.69, P = 0.04) and the Bdellovibrionata order 0319-6G20 (LDA score = 4.27, P = 0.01) were significantly more characteristic of the cryosphere than lake and river water (Supplementary Table S6), and Deinococcota were overrepresented in Thores Glacier ice and cryoconite water. In these same samples, up to 50% of reads belonged to Cyanobacteria (Fig. 4c, Supplementary Fig. S1 & S2) and were partitioned by ice type: the filamentous, biofilm-associated taxa Leptolyngbya and Pseudanabaena dominated the glacier samples (Supplementary Fig. S1), while the bottom of lake ice contained only planktonic Cyanobium, suggesting its origin from lake water freeze-up. Surface lake ice (in contact with air) on the other hand contained a large proportion of sequences whose taxonomy at the phylum level could not be assigned (16.3%, Fig. 4c). Waterfalls flowing from Thores Glacier, and from a second, unnamed glacier 6.3 km downstream into Thores River were similar to each other in composition (Fig. 4d), but distinct from the glacier ice and cryoconites: both waterfalls had more Comamonadaceae (Proteobacteria) and Pseudanabaenaceae (Cyanobacteria) than ice (Supplementary Fig. S2).
a Relative abundance of photosynthetic organisms based on their oxygen regime. b Sample richness (from Shannon’s diversity index). c Phylum-level relative abundance (top dataset-wide phyla shown, the rest grouped in “Others”). Colors show habitat type – when a sample was taken at the interface of two categories, both colors are represented. d Principal coordinate analyses from Bray-Curtis dissimilarity matrix showing beta diversity across all samples.
Distinct community composition in downstream aquatic habitats
The planktonic communities in Thores Lake differed strikingly from the glacial communities (Fig. 4c, d). The reads were dominated by Ilumatobacteraceae and Sporychtyaceae (Actinobacteriota), Anaerolineaeceae (Chloroflexi), Gemmataceae, Phycisphaeraceae and Pirellulaceae (Planctomycetota) (Fig. 5, Supplementary Fig. S2) and were homogenous to 40 m, consistent with the lack of thermal or oxic stratification in Thores Lake in the sampling depth range. Comamonadaceae (including Polaromonas) were also consistently present throughout the water column (average of 9.1% of reads; Fig. 5). Bacterial phototrophs in the water column were mostly associated with anoxygenic photosynthesis (Choroflexi), contrary to glacier prokaryotes which were overwhelmingly linked to oxygenic photosynthesis (Cyanobacteria) (Fig. 4a). However, oxygenic Synechococcales were detected in low relative abundance in the water column, and were the only representatives of Cyanobacteria in the lake (Fig. 5 & Supplementary Fig. S1).
The ice-contact zone with its unstable ice cover and high turbidity connecting the glacier and lake (hereafter referred to as the interface) was dominated by Proteobacteria (Fig. 4c), and was the most diverse site across the Thores glacier-lake-outflow continuum (Fig. 4b, 981 taxa), with a mixture of glacial and lake water representatives. Near its outflow from the lake, the Thores River community was similar to the lake plankton assemblage, and this community composition persisted 16 km downstream at its entry point to Disraeli Fiord, but with an increased representation of Comamonadaceae (Proteobacteria), and a decreasing proportion of unassigned taxa (Supplementary Fig. S2).
Bacterial connectivity between habitats
Habitat type explained 46.3% of variation in community composition (adonis P = 0.001, F = 4.739, degrees of freedom = 2), with cryosphere-associated samples clustering along Axis 1, except for the bottom of lake ice (Fig. 4d). The interface was distinct from the glacier and water column, and with some resemblance to lake ice (Fig. 4d), had the greatest taxonomic richness (Fig. 4b) and no unique Polaromonas phylotypes, unlike the glacier and lake (Fig. 2b). While samples were collected over two summers using different filter sizes (0.22 and 0.02 µm) and corresponding nucleic acid extraction protocols (see Methods), filter type contributed only marginally to explaining community assemblage (21% variation, P = 0.01) (Supplementary Fig. S3).
Source tracking identified 47% of taxa in the glacier-lake interface as originating from lake ice (Fig. 6, Supplementary Table S7), but the origin of most taxa in this sample could not be determined. However, the proportion of identifiable taxa rose to 90% in Thores River. The lake water column and ice cover appeared to influence one another: 87–93% of water column microbes were tracked to ice, and 62.6% of microbes in the bottom layers of lake ice were sourced to the water column below (Fig. 6 & Supplementary Table S7). Surface lake ice was more impacted by the glacier (91.9%), and little seeded by the water column at the time of sampling (0.6%) (Fig. 6 & Supplementary Table S7). At the head of Thores River, approximately 88% of taxa were from the lake, and at its mouth near Disraeli Fiord, 16 km downstream, 32% of taxa could still be tracked to Thores Lake.
Potential sources are the glacier, lake ice, and lake water. Taxa whose origin could not be traced to these categories are marked as “Unknown”. Sinks across the continuum include the interface, lake ice (surface and bottom), lake water (at 5 m), the mouth of the outflow, and its junction in Disraeli Fiord 16 km downstream. Pie charts display the proportion of the origin of microbial taxa in each sink, and yellow arrows show the direction of exchanges from sources to sinks across the continuum. Circled numbers identify sinks on the illustrations. The mean source tracking values and confidence intervals for all sinks (including the rest of the water column) are given in Supplementary Table S7.
Discussion
Our observations from the LIA terrestrial margin draw attention to the rich diversity of cryohabitats and bacterial communities within this region and their vulnerability to climate change. The genus Polaromonas was found across almost all habitat types, and consistent with our hypothesis of genetic divergence among niches, there were pronounced differences in Polaromonas phylotypes between the ice and water environments. This niche specificity extended to the overall communities, but contrary to our expectation of hydrological connectivity as a dominant force in downstream assembly processes, the bacterial dominants diverged greatly between the glacier and the main body of the lake. The ice margin interface had its own distinctive microbiome, with a mixture of the lake and glacial Polaromonas phylotypes, but also with the highest diversity of other bacterial taxa, including many that were not shared upstream with the glacier nor downstream in proglacial Thores Lake and its outflow.
Cold-dwelling Comamonadaceae are widely distributed in the Arctic, including in thermokarst thaw ponds [52] and in Lake Hazen [53], a large lake on Ellesmere Island that lies 100 km southeast of Thores Lake. Polaromonas was the most notable member of the Comamonadaceae family in this study, and one Polaromonas phylotype was the only taxon present in all samples in the dataset (at a 1% relative abundance threshold). This genus is ubiquitous in the polar regions and other cold environments [13] and has an especially high potential for dispersal [11], consistent with the presence of shared Polaromonas ASVs between habitats in the Thores glacier-lake-river continuum.
Many of the Polaromonas phylotypes identified in Thores were generalists, i.e. organisms that appear to survive under diverse conditions, consistent with the metabolic versatility found within this genus [54]. This includes phylotype pol2, which was detected throughout the watershed, with the greatest relative abundances at the junction of Thores River in Disraeli Fiord. Phylotype pol1, which was the most abundant member of the Polaromonas genus in Thores, was also ubiquitous. This phylotype was closely related to P. ginsengisoli, which was initially isolated from soil, and shares 98.6% of its 16 S rRNA with P. eurypsychrophila [55], but has since been identified in other systems, including in the water column of a boreal lake during winter [56]. The consistent presence of these two phylotypes (pol1, pol2) across the watershed suggests these members of the Polaromonas genus are generalists with broad tolerances that likely endure year-to-year, as data presented here were collected over two consecutive summers.
Phylotypes pol3 & pol5 were consistently found in the lake, as well as in lesser abundances in other compartments downstream of the lake. These organisms were closely related to P. eurypsychrophila, which was originally isolated in glacier ice on the Tibetan Plateau [57]. These phylotypes were either not detected, or present in low abundances in Thores glacier ice. Different drivers of niche-partitioning of Polaromonas have been suggested to operate on glacial surfaces, including sorting by local habitat conditions, but also community interactions with other prokaryotes and with eukaryotic microbes [13], which may also be at play in free-flowing waters in the Thores system.
The lack of Polaromonas phylotypes specific to the glacier-lake interface implies that this is a mixing and transition zone, with insufficient time or selective forces to result in detectable genetic differentiation within this taxon. A limnological study of Thores Lake showed that this zone has many distinct properties, including in nutrients, conductivity and turbidity [14]. Consistent with these features and with the many bacterial ASVs (but not Polaromonas) that were observed in the present study to be unique to this zone, the interface also contained a distinct phytoplankton community, with a higher proportion of green algae and the chlorophyte pigment chlorophyll b relative to the main body of the lake, which was characterized by chrysophytes and fucoxanthin [15]. We found that the interface community lacked the filamentous cyanobacteria Leptolyngbya and Pseudanabaena that dominated the glacier samples, indicating strong selection against them, or reduced dispersal abilities of these biofilm-forming taxa. As sampling occurred over two different summers, there may be inter- or intra-year variation patterns that were not observed. However, Cyanobacteria have been found to exhibit long-term persistence in perennially cold habitats, suggesting robust assemblages on the glacier surface [58].
The presence of both lake and glacier Polaromonas in the mixing zone suggests that these phylotypes have sufficiently broad tolerances to persist in such conditions, and to resist microbial loss processes during their short residence time in the transition zone, while other groups like Cyanobacteria that originate from the glacier are more likely to be filtered in the interface.
Source tracking has been used in many instances to model microbial connectivity across glaciers [8, 59, 60], and has been applied in nearby Ward Hunt Lake [10], where snow was found to be a major seeder of microbes to the lake, including Polaromonas in the littoral mixing zone. In the present study, we were unable to identify the source of most taxa, especially in the lake, and the percentage of taxa whose source was identified was lower than at Ward Hunt. This may be due to the long term stability of Thores Glacier [16] and its specialized microbiome, or to the longer residence time of Thores Lake [15] that allows sufficient time for in-lake processes to shape the communities.
The strong in-lake effect on microbiome composition was indicated not only by the unique Polaromonas phylotypes in Thores Lake but also by the distinctive assemblage of other taxa. Actinobacteriota accounted for approximately 20% of reads throughout the water column; cells of this phylum are typically small in size and are often abundant in oligotrophic freshwaters [61]. Planctomycetota (average of 19.8% of lake reads) has also been identified as an important feature of ice-covered lakes at the far northern coast of Ellesmere Island [62], and one of the main planctomycete families in Thores Lake, the Gemmataceae, is known to include psychrotolerant taxa [63]. The high relative abundance (13%) of Anaerolineales (Phylum Chloroflexi) is also unusual for most freshwater ecosystems and implies the importance of anoxygenic aerobic phototrophy in Thores Lake. This class has been identified among the dominants in deep, cold, oxygenated hypolimnia, including in Lake Biwa [64], Japan, and Crater Lake [65], USA. These distinctive microbiome features of Thores Lake also differed markedly from High Arctic Lake Hazen, where ice has a less pervasive influence on habitat structure. Polaromonas was only detected at the bottom of Lake Hazen where glacial meltwater joins the water column [53], whereas this genus was ubiquitous throughout Thores Lake. Furthermore, while Chloroflexi were an important feature of Lake Hazen in spring at comparable relative abundances to Thores Lake, they decreased over summer [53]; and Bacteroidota, the second most abundant phylum in Lake Hazen, accounted for less than 6% of reads in the Thores water column.
Another cryospheric compartment of interest in the Thores Lake system is the perennial ice cover, which would protect the lake from direct airborne seeding. There are likely, however, to be complex interactions between the lake ice and the underlying lake waters. While dissolved compounds (organic carbon, phosphorus) are excluded from lake ice during its formation, particles can be retained in the new ice [66]. This freeze-trapping effect may explain the presence of Cyanobium in the bottom of Thores Lake ice since this picocyanobacterial genus was abundant in the surface waters of the lake.
The results obtained here underscore the high bacterial species richness in the Thores ice-water ecosystem, and the diverse array of community assemblages that segregate among cryohabitats. The presence of ASVs does not necessarily imply that the cells are actively growing, but transcriptomic studies of glacier microbiomes elsewhere have indicated that a large proportion of the observed community has the potential for metabolic activity [67]. However, our analyses captured only a subset of the total microbial diversity, and studies at other ice-associated sites in the LIA margin have drawn attention to diverse assemblages of archaea [68], viruses [69, 70], and microbial eukaryotes, including new fungal taxa [71]. Microbial surveys of cryohabitats in other regions have shown the importance of desmid blooms on ice caps [72] and snow algal growth on glaciers [73]. These components are likely to be similarly diverse in the Thores ecosystem, and to be similarly vulnerable to any habitat loss that accompanies ongoing climate warming in the region. While it is challenging to obtain high-resolution data in systems as remote as Thores, this study provides a snapshot of the microbial assemblages of a rare, stable glacier-lake coupled system in the High Arctic. There is a pressing need for further research to increase the spatial and temporal coverage of microbiome studies in polar environments.
Arctic ice-dwelling microbes are at the forefront of climate change, and are threatened by habitat extinction. There is therefore an urgent need to understand this unique biodiversity before it is lost to further warming [74, 75]. Microbes may also accelerate the melting of ice in warmer conditions, by reducing albedo and promoting ablation of glaciers, snow, and ice shelves [73, 76]. Shrinking of the cryosphere will increase the dispersal of microbes across Arctic landscapes [77,78,79], and while some microbes will be driven to extinction by the environmental conditions in their new habitats, cold-adapted Polaromonas may evolve into novel ecotypes, potentially losing cold-adaptation functions and genetic diversity.
The Last Ice Area is projected to remain ice-covered into the middle of the century when summer ice will have largely disappeared from the rest of the Arctic Ocean [1]. The perennially cold terrestrial margin will therefore be an enduring refuge for ice-dependent microbes in the coastal High Arctic. However, proglacial ecosystems such as the Thores glacier-lake-outflow continuum are entirely dependent on the integrity of the glacial ice, and loss of the glacier would result in complete drainage and loss of the lake and its associated environments [16]. Cryohabitats and their rich microbiota, including specialized ecotypes of Polaromonas, will likely persist in this refuge into the immediate future, but will be prone to more severe effects of ongoing climate change.
Data availability
Raw amplicon sequences are available at the NCBI public database under BioProject ID PRJNA905405.
References
Newton R, Pfirman S, Tremblay LB, DeRepentigny P. Defining the “Ice Shed” of the Arctic Ocean’s last ice area and its future evolution. Earth’s Future. 2021;9:e2021EF001988. https://doi.org/10.1029/2021EF001988
Vincent WF, Mueller D. Witnessing ice habitat collapse in the Arctic. Science. 2020;370:1031–2. https://doi.org/10.1126/science.abe4491
Copland L, Mueller D. Arctic Ice Shelves and Ice Islands. New York, NY: Springer Berlin Heidelberg; 2017.
Bégin PN, Tanabe Y, Rautio M, Wauthy M, Laurion I, Uchida M, et al. Water column gradients beneath the summer ice of a High Arctic freshwater lake as indicators of sensitivity to climate change. Sci Rep. 2021;11:1–12. https://doi.org/10.1038/s41598-021-82234-z
Boetius A, Anesio AM, Deming JW, Mikucki JA, Rapp JZ. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat Rev Microbiol. 2015;13:677–90. https://doi.org/10.1038/nrmicro3522
Di Mauro B, Garzonio R, Baccolo G, Franzetti A, Pittino F, Leoni B, et al. Glacier algae foster ice-albedo feedback in the European Alps. Sci Rep. 2020;10:1–9. https://doi.org/10.1038/s41598-020-61762-0
Anesio AM, Bellas CM. Are low temperature habitats hot spots of microbial evolution driven by viruses? Trends Microbiol. 2011;19:52–7. https://doi.org/10.1016/j.tim.2010.11.002
Cameron KA, Müller O, Stibal M, Edwards A, Jacobsen CS. Glacial microbiota are hydrologically connected and temporally variable. Environ Microbiol. 2020;22:3172–87. https://doi.org/10.1111/1462-2920.15059
Skladnev DA, Mulyukin AL, Filippova SN, Kulikov EE, Letarova MA, Yuzbasheva EA, et al. Modeling of dissemination of microbial cells and phages from the sites of permafrost thawing. Microbiology. 2016;85:614–9. https://doi.org/10.1134/S0026261716050167
Comte J, Culley AI, Lovejoy C, Vincent WF. Microbial connectivity and sorting in a High Arctic watershed. ISME J. 2018;12:2988–3000. https://doi.org/10.1038/s41396-018-0236-4
Darcy JL, Lynch RC, King AJ, Robeson MS, Schmidt SK. Global distribution of Polaromonas phylotypes - evidence for a highly successful dispersal capacity. PLoS ONE. 2011;6:e23742. https://doi.org/10.1371/journal.pone.0023742
Ciok A, Budzik K, Zdanowski MK, Gawor J, Grzesiak J, Decewicz P, et al. Plasmids of psychrotolerant Polaromonas spp. isolated from Arctic and Antarctic glaciers – diversity and role in adaptation to polar environments. Front Microbiol. 2018;9:1285. https://doi.org/10.3389/fmicb.2018.01285
Gawor J, Grzesiak J, Sasin-Kurowska J, Borsuk P, Gromadka R, Górniak D, et al. Evidence of adaptation, niche separation and microevolution within the genus Polaromonas on Arctic and Antarctic glacial surfaces. Extremophiles. 2016;20:403–13. https://doi.org/10.1007/s00792-016-0831-0
Saulnier-Talbot É, Duchesne E, Antoniades D, Arseneault D, Barnard C, Berteaux D, et al. State shifts and divergent sensitivities to climate warming across northern ecosystems. Res Square. 2023. https://doi.org/10.21203/rs.3.rs-3026284/v1
Culley AI, Thaler M, Kochtitzky W, Iqaluk P, Rapp JZ, Rautio M, et al. The Thores Lake proglacial system: remnant stability in the rapidly changing High Arctic. Arctic Sci n.d.;In press. https://doi.org/10.1139/AS-2022-0023
Kochtitzky W, Copland L, Wohlleben T, Iqaluk P, Girard C, Vincent WF, et al. Slow change since the Little Ice Age at a far northern glacier with the potential for system reorganization: Thores Glacier, northern Ellesmere Island, Canada. Arctic Sci n.d.;In press. https://doi.org/10.1139/as-2022-0012
Carrivick JL, Tweed FS, Sutherland JL, Mallalieu J. Toward numerical modeling of interactions between ice-marginal proglacial lakes and glaciers. Front Earth Sci. 2020;8:577068. https://doi.org/10.3389/feart.2020.577068
Bégin PN, Tanabe Y, Kumagai M, Culley AI, Paquette M, Sarrazin D, et al. Extreme warming and regime shift toward amplified variability in a far northern lake. Limnol Oceanogr. 2020;66:S17–29. https://doi.org/10.1002/lno.11546
Wetzel RG, Likens GE. Limnological analyses. 3rd edition. Springer Press; 2000.
Patton CJ, Kryskalla JR. Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory – Evaluation of alakaline persulfate digestion as an alternative to Kjedahl digestion for determmination of total and dissolved nitrogen and phosphorus. U.S. Geological Survey; 2003.
Cuthbert ID, del Giorgio P. Toward a standard method of measuring color in freshwater. Limnol Oceanogr. 1992;37:1319–26.
Wauthy M, Rautio M. Permafrost thaw stimulates primary producers but has a moderate effect on primary consumers in subarctic ponds. Ecosphere. 2020;11:e03099. https://doi.org/10.1002/ecs2.3099
Thaler M, Vincent WF, Lionard M, Hamilton AK, Lovejoy C. Microbial community structure and interannual change in the last epishelf lake ecosystem in the north polar region. Front Mar Sci. 2017;3:275. https://doi.org/10.3389/fmars.2016.00275
Culley AI, Girard C, Vincent WF. Physico-chemical profiles in proglacial Thores Lake, Ellesmere Island, Nunavut, v. 1.1. Nordicana D82 2022. https://doi.org/10.5885/45669CE-3078BA69AC9944CA
Girard C, Rautio M, Culley AI, Vincent WF. Plankton and water chemistry data from proglacial Thores Lake, v. 1.0. Nordicana D105 2022. https://doi.org/10.5885/45801CE-647F4C260BD64AEC
Cruaud P, Vigneron A, Fradette M-S, Charette SJ, Rodriguez MJ, Dorea CC, et al. Open the SterivexTM casing: an easy and effective way to improve DNA extraction yields. Limnol Oceanogr Methods. 2017;15:1015–20. https://doi.org/10.1002/lom3.10221
Mueller JA, Culley AI, Steward GF. Variables influencing extraction of nucleic acids from microbial plankton (viruses, bacteria, and protists) collected on nanoporous aluminum oxide filters. Appl Environ Microbiol. 2014;80:3930–42. https://doi.org/10.1128/AEM.00245-14
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. https://doi.org/10.1038/nmeth.3869
R Core Team. R: A language and environment for statistical computing 2017.
Callahan BJ, McMurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017;11:2639–43. https://doi.org/10.1038/ismej.2017.119
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–6. https://doi.org/10.1093/nar/gks1219
McMurdie PJ, Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. https://doi.org/10.1371/journal.pone.0061217
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community ecology package. R package version 2.5-6 2019.
Wickham H. stringr: simple, consisten wrappers for common string operations 2019.
Wright ES. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 2016;8:352. https://doi.org/10.32614/RJ-2016-025
Wickham H, François R, Henry L, Müller K. dplyr: a grammer of data manipulation 2020.
Wickham H, Henry L. tidyr: tidy messy data 2020.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlang; 2016.
Neuwirth E. RColorBrewer: ColorBrewer palettes 2014.
Lahti L, Shetty S. microbiome R package. 2012.
Cardona T. A fresh look at the evolution and diversification of photochemical reaction centers. Photosynth Res. 2015;126:111–34. https://doi.org/10.1007/s11120-014-0065-x
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Cao Y. microbiomeMarker: microbiome biomarker analysis 2020.
Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–40. https://doi.org/10.1093/bioinformatics/btx364
Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, et al. Bayesian community-wide culture-independent microbial source tracking. Nat Methods. 2011;8:761–3. https://doi.org/10.1038/nmeth.1650
Madeira F, Park Ymi, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–41. https://doi.org/10.1093/nar/gkz268
Hwang K, Choe H, Nasir A, Kim KM. Complete genome of Polaromonas vacuolata KCTC 22033T isolated from beneath Antarctic Sea ice. Mar Genomics. 2021;55:100790. https://doi.org/10.1016/j.margen.2020.100790
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–9. https://doi.org/10.1093/nar/gkz239
Nakamura T, Yamada KD, Tomii K, Katoh K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics. 2018;34:2490–2. https://doi.org/10.1093/bioinformatics/bty121
Stecher G, Tamura K, Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol. 2020;37:1237–9. https://doi.org/10.1093/molbev/msz312
Gouy M, Guindon S, Gascuel O. SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–4. https://doi.org/10.1093/molbev/msp259
Comte J, Lovejoy C, Crevecoeur S, Vincent WF. Co-occurrence patterns in aquatic bacterial communities across changing permafrost landscapes. Biogeosciences. 2016;13:175–90. https://doi.org/10.5194/bg-13-175-2016
Cavaco MA, St. Louis VL, Engel K, St. Pierre KA, Schiff SL, Stibal M, et al. Freshwater microbial community diversity in a rapidly changing High Arctic watershed. FEMS Microbiol Ecol. 2019;95:fiz161. https://doi.org/10.1093/femsec/fiz161
Yagi JM, Sims D, Brettin T, Bruce D, Madsen EL. The genome of Polaromonas naphthalenivorans strain CJ2, isolated from coal tar-contaminated sediment, reveals physiological and metabolic versatility and evolution through extensive horizontal gene transfer. Environ Microbiol. 2009;11:2253–70. https://doi.org/10.1111/j.1462-2920.2009.01947.x
Choi KD, Siddiqi MZ, Liu Q, Jo JH, Chun SY, Choi G-M, et al. Polaromonas ginsengisoli sp. nov., isolated from ginseng field soil. Int J Syst Evol. 2018;68:1436–41. https://doi.org/10.1099/ijsem.0.002669
Laanto E, Oksanen HM. Three phages from a boreal lake during ice cover infecting Xylophilus, Caulobacter, and Polaromonas species. Viruses. 2023;15:307. https://doi.org/10.3390/v15020307
Xing T, Yao T, Liu Y, Wang N, Xu B, Shen L, et al. Polaromonas eurypsychrophila sp. nov., isolated from an ice core. Int J Syst Evol Microbiol. 2016;66:2497–501. https://doi.org/10.1099/ijsem.0.001079
Quesada A, Vincent WF. Cyanobacteria in the cryosphere: snow, ice and extreme cold. In: Whitton B, editor. Ecology of Cyanobacteria II. Dordrecht: Springer; 2012.
Peter H, Sommaruga R. Shifts in diversity and function of lake bacterial communities upon glacier retreat. ISME J. 2016;10:1545–54. https://doi.org/10.1038/ismej.2015.245
Weisleitner K, Perras A, Moissl-Eichinger C, Andersen DT, Sattler B. Source environments of the microbiome in perennially ice-covered Lake Untersee, Antarctica. Front Microbiol. 2019;10:1019. https://doi.org/10.3389/fmicb.2019.01019
Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F. Metagenomics uncovers a new group of low GC and ultra-small marine Actinobacteria. Sci Rep. 2013;3:2471. https://doi.org/10.1038/srep02471
Marois C, Girard C, Klanten Y, Vincent WF, Culley AI, Antoniades D. Local habitat filtering shapes microbial community structure in four closely spaced lakes in the High Arctic. Front Microbiol. 2022;13:779505. https://doi.org/10.3389/fmicb.2022.779505
Kulichevskaya IS, Ivanova AA, Naumoff DG, Beletsky AV, Rijpstra WIC, Sinninghe Damsté JS, et al. Frigoriglobus tundricola gen. nov., sp. nov., a psychrotolerant cellulolytic planctomycete of the family Gemmataceae from a littoral tundra wetland. Syst Appl Microbiol. 2020;43:126129. https://doi.org/10.1016/j.syapm.2020.126129
Okazaki Y, Hodoki Y, Nakano S. Seasonal dominance of CL500-11 bacterioplankton (phylum Chloroflexi) in the oxygenated hypolimnion of Lake Biwa, Japan. FEMS Microbiol Ecol. 2013;83:82–92. https://doi.org/10.1111/j.1574-6941.2012.01451.x
Urbach E, Vergin KL, Young L, Morse A, Larson GL, Giovannoni SJ. Unusual bacterioplankton community structure in ultra‐oligotrophic Crater Lake. Limnol Oceanogr. 2001;46:557–72. https://doi.org/10.4319/lo.2001.46.3.0557
Imbeau E, Vincent WF, Wauthy M, Cusson M, Rautio M. Hidden stores of organic matter in Northern lake ice: selective retention of terrestrial particles, phytoplankton and labile carbon. J Geophys Res Biogeosci. 2021;126:e2020JG006233. https://doi.org/10.1029/2020JG006233
Bradley JA, Trivedi CB, Winkel M, Mourot R, Lutz S, Larose C, et al. Active and dormant microorganisms on glacier surfaces. Geobiology 2022:gbi.12535. https://doi.org/10.1111/gbi.12535
Vigneron A, Cruaud P, Lovejoy C, Vincent WF. Genomic evidence of functional diversity in DPANN archaea, from oxic species to anoxic vampiristic consortia. ISME Commun. 2022;2:1–10. https://doi.org/10.1038/s43705-022-00088-6
Labbé M, Girard C, Vincent WF, Culley AI. Extreme viral partitioning in a marine-derived High Arctic lake. MSphere. 2020;5:e00334–20. https://doi.org/10.1128/mSphere.00334-20
Labbé M, Thaler M, Pitot TM, Rapp JZ, Vincent WF, Culley AI. Climate-endangered Arctic epishelf lake harbors viral assemblages with distinct genetic repertoires. Appl Environ Microbiol. 2022;88:e00228–22. https://doi.org/10.1128/aem.00228-22
Tsuji M, Vincent WF, Tanabe Y, Uchida M. Glacier retreat results in loss of fungal diversity. Sustainability. 2022;14:1617. https://doi.org/10.3390/su14031617
Williamson CJ, Turpin-Jelfs T, Nicholes MJ, Yallop ML, Anesio AM, Tranter M. Macro-nutrient stoichiometry of glacier algae from the southwestern margin of the Greenland Ice Sheet. Front Plant Sci. 2021;12:673614. https://doi.org/10.3389/fpls.2021.673614
Lutz S, Anesio AM, Raiswell R, Edwards A, Newton RJ, Gill F, et al. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nat Commun. 2016;7:1–9. https://doi.org/10.1038/ncomms11968
Vincent WF. Microbial ecosystem responses to rapid climate change in the Arctic. ISME J. 2010;4:1087–90. https://doi.org/10.1038/ismej.2010.108
Edwards A, Cameron KA, Cook JM, Debbonaire AR, Furness E, Hay MC, et al. Microbial genomics amidst the Arctic crisis. Microb Genomics. 2020;6:e000375. https://doi.org/10.1099/mgen.0.000375
Halbach L, Chevrollier L-A, Doting EL, Cook JM, Jensen MB, Benning LG, et al. Pigment signatures of algal communities and their implications for glacier surface darkening. Sci Rep. 2022;12:1–14. https://doi.org/10.1038/s41598-022-22271-4
Hotaling S, Hood E, Hamilton TL. Microbial ecology of mountain glacier ecosystems: biodiversity, ecological connections and implications of a warming climate. Environ Microbiol. 2017;19:2935–48. https://doi.org/10.1111/1462-2920.13766
Paquette M, Fortier D, Vincent WF. Water tracks in the High Arctic: a hydrological network dominated by rapid subsurface flow through patterned ground. Arctic Sci. 2017;3:334–53. https://doi.org/10.1139/as-2016-0014
Harding T, Jungblut AD, Lovejoy C, Vincent WF. Microbes in High Arctic snow and implications for the cold biosphere. Appl Environ Microbiol. 2011;77:3234–43. https://doi.org/10.1128/AEM.02611-10
Acknowledgements
We are grateful to the community of Resolute Bay (Qausuittuq) for their support of this research, and to the Nunavut Research Institute for authorizing this work. We thank Parks Canada for use of their facilities at Quttinirpaaq National Park and for their support of research in the park, to the Polar Continental Shelf Program (National Ressources Canada) for logistical support and to Parks Canada and the Center for Northern Studies (CEN, Université Laval) for their infrastructure on Ward Hunt Island. The authors are also grateful to Pilipoosie Iqaluk, Elise Imbeau, Will Kochtitzky, Denis Sarrazin, Yukiko Tanabe and Derek Mueller for support and assistance on the field. We also thank Juliette Provencher, Carlee Morency, Anne-Marie Lapointe, Catherine Marois and Brian Boyle for molecular lab work and sequencing, and Milla Rautio and her group for chemical analyses. The authors thank Elise Imbeau for illustrations in Figs. 1 and 6. We acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Fonds de recherche du Québec-Nature et technologie (FRQNT), the Network of Centres of Excellence of Canada ArcticNet and the Canada First Research Excellence Fund (CFREF) program Sentinel North. CG was supported by postdoctoral fellowship awards from the FRQNT and Sentinel North.
Author information
Authors and Affiliations
Contributions
Conceptualization CG, WFV, AIC. Data curation: CG. Formal analysis: CG. Investigation and project administration: CG, WFV, AIC. Writing, review and editing: CG, WFV, AIC. Funding: WFV, AIC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Girard, C., Vincent, W.F. & Culley, A.I. Arctic bacterial diversity and connectivity in the coastal margin of the Last Ice Area. ISME COMMUN. 3, 105 (2023). https://doi.org/10.1038/s43705-023-00313-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43705-023-00313-w